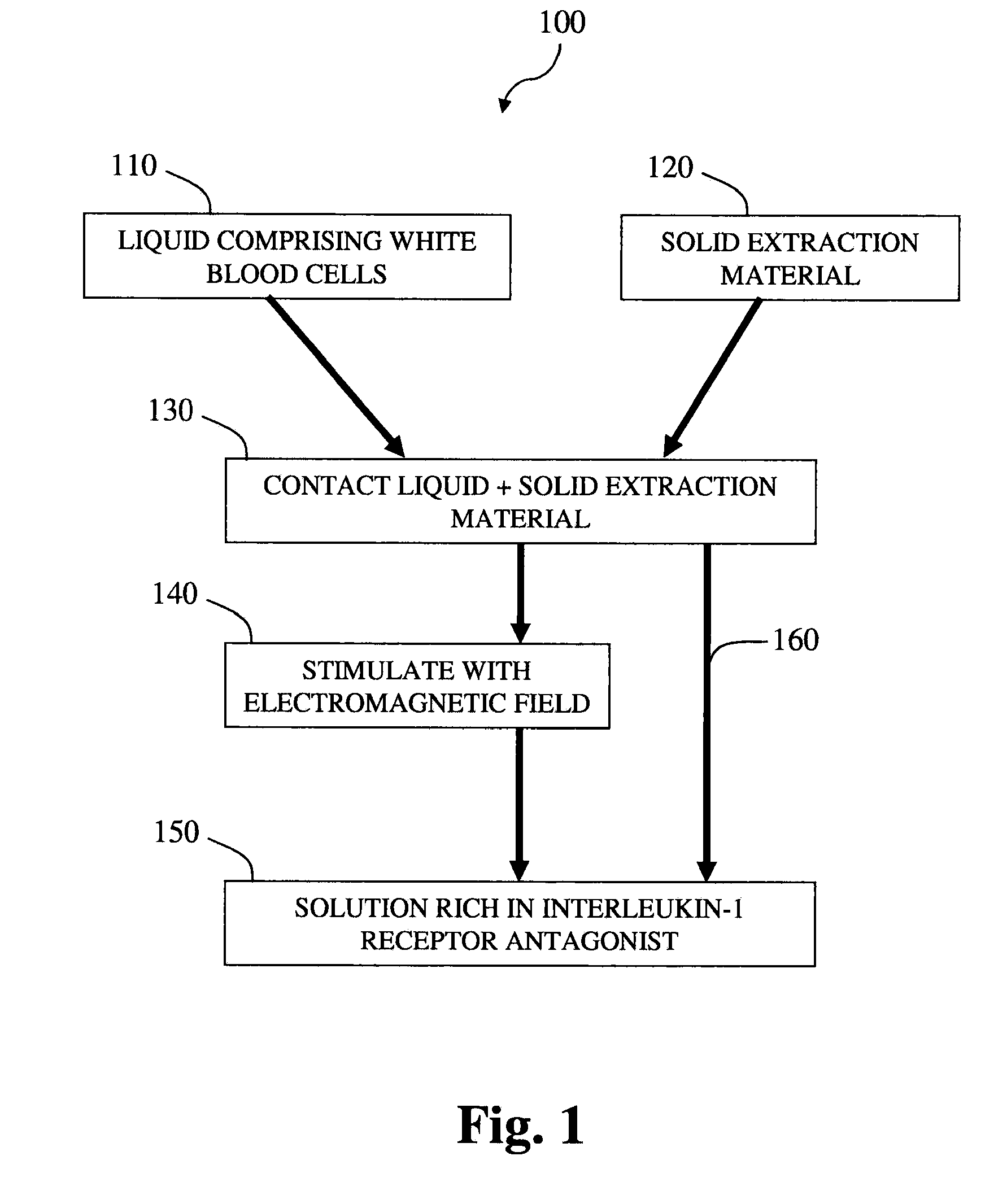
Methods and compositions for delivering interleukin-1 receptor antagonist
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Characterization of Solutions Rich in IL-1ra
[0123]A solution rich in interleukin-1 receptor antagonist was prepared from seven consented human providers. Blood (55 mL) was drawn into a 60 cc syringe with 5 mL of anticoagulant citrate dextrose solution A (ACD-A, Citra Anticoagulant, Inc., Braintree, Mass.). Platelet-rich plasma (PRP) was created using the GPS III platelet concentration system (800-1 003A, Biomet Biologics, Warsaw, Ind.) according to the instructions for use. The solution was generated by adding 6 mL of PRP to a modified Plasmax device containing 1 gram of polyacrylamide beads (Biomet Biologics, Warsaw, Ind.). The IL-1ra solution was removed from the Plasmax devices and was frozen at minus 50° C. for the assay. Cytokine content was assayed on a 16-plex ELISA (Searchlight Protein Array, Aushon Biosystems, Billerica, Mass.). The analytes included IL-4, IL-10, IL-11, IL-13, IL-1ra, IFN-Δ, sTNF-RI, sTNF-RII, IL-1α, IL-1β, TNF-α, IL-17, IL-18, bFGF, TBF-β1, and TBF-β2.
[012...
example 2
A Solution Rich in Interleukin-1 Receptor Antagonist Made from Equine Blood
[0125]A solution rich in interleukin-1 receptor antagonist was prepared from equine blood. Platelet-rich plasma (PRP) was created using the GPS 11I platelet concentration system (8001003A, Biomet Biologics, Warsaw, Ind.) according to the instructions for use. The solution was generated by adding 6 mL of PRP to a modified Plasmax device containing I gram of polyacrylamide beads (Biomet Biologics, Warsaw, Ind.). The IL-1ra solution was removed from the Plasmax devices and was frozen at minus 50° C. for an ELISA assay (Equine DuoSet ELISA kit, R&D Systems, Minneapolis, Minn.). Equine IL-1ra was measured in the baseline whole blood, PRP, and IL-1ra solution. The devices used were able to produce a solution rich in interleukin-1 receptor antagonist (FIG. 12).
example 3
Generation of IL-1ra from Platelet-Rich Plasma
[0126]An IL-1ra-rich solution is created as follows. Whole blood (70 mL) anticoagulated (10%) with ACD-A (Braintree, Mass., USA) is drawn from 5 healthy volunteers. A portion (10 mL) is reserved for a whole blood measurement. Platelet-rich plasma (PRP) (6 mL) is produced using the GPS® II System (Biomet Biologics, LLC, Warsaw, Ind., USA). Complete blood counts are collected for the whole blood and PRP samples following a validated procedure, as described in Woodell-May J E, Ridderman D N, Swift M J, Higgins J. “Producing Accurate Platelet Counts for Platelet Rich Plasma: Validation of a Hematology Analyzer and Preparation Techniques for Counting”J. Craniofac. Surg. (2005) September 16(5):749-56.
[0127]Following the PRP production, 5 mL of the PRP is added to a modified plasma concentration device (Plasmax™, Biomet Biologics LLC, Warsaw, Ind., USA) and incubated with polyacrylamide desiccating beads in the device for 24 hours at room tempe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com