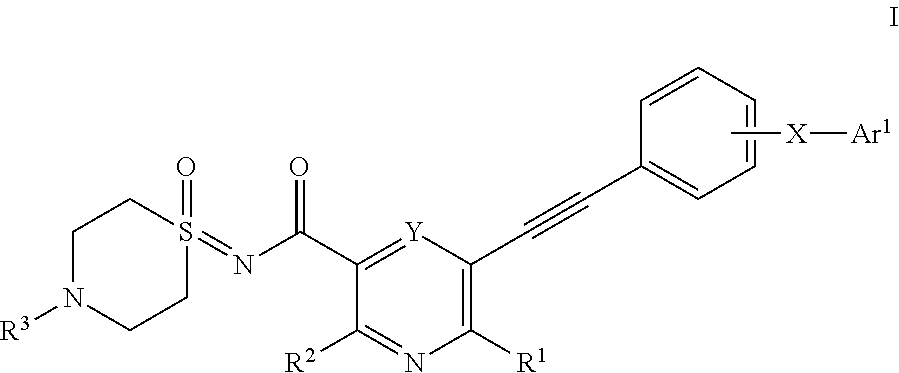
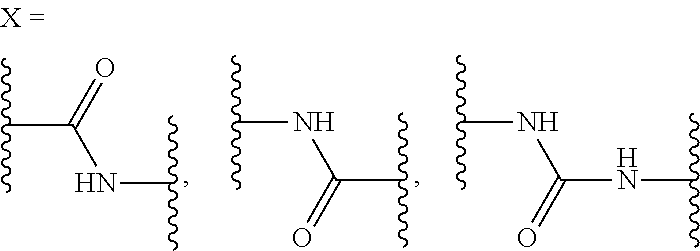
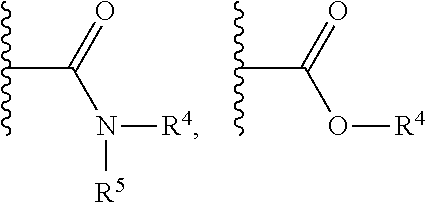
Substituted nicotinamide derivatives as kinase inhibitors
a technology of nicotinamide and derivatives, which is applied in the direction of drug compositions, immunological disorders, metabolism disorders, etc., can solve the problems of abnormal blood vessel growth, malignant tumor growth, or defects in key developmental processes, and achieve the effect of modulating, regulating and/or inhibiting tyrosine kinase signal transduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation 1
[0068]
tert-Butyl thiomorpholine-4-carboxylate
[0069]To a solution of thiomorpholine (4.84 mL, 50 mmol, 1 eq) in DCM (200 mL) was added Et3N (14.6 mL, 2.1 eq) and di-tert-butyldicarbonate (12.0 g, 1.1 eq) with stirring under nitrogen atmosphere. The resulting clear solution was stirred at RT for an overnight. The reaction solution was washed with H2O (1×), aqueous NH4Cl (1×), brine (1×) and dried over anhydrous MgSO4. The organic solution was filtered through a pad of celite and the filtrate concentrated. The white solid residue was treated with EtOAc-hexane (1:25) with stirring and then cooled in fridge for 30 min. The white solid which formed was collected to give the title compound as a white crystalline solid (10.1 g, quantitative). 1H NMR (DMSO-d6) δ: 3.54-3.59 (m, 4H), 2.48-2.54 (m, 4H), 1.40 (s, 9H)
preparation 2
[0070]
tert-Butyl thiomorpholine-4-carboxylate 1-oxide
[0071]In a 250 mL round-bottom flask equipped with a magnetic stirrer were placed powdered sodium metaperiodate (5.68 g, 1.05 eq) and water (50 mL). The mixture was stirred at RT first and then cooled to 0° C., followed by the addition of tert-butyl thiomorpholine-4-carboxylate (5.08 g, 25 mmol, 1 eq). Then to this mixture was added dixane (30 mL) and MeOH (40 mL). The reaction mixture was stirred at 0° C. for 5.5 hours. It was then filtered through a Buchner funnel, the white solid was washed with CHCl3 (3λ50 mL), and the resulting water-chloroform filtrate was transferred into a separation funnel. The lower chloroform was removed and the aqueous layer was extracted with CHCl3 (3×150 mL). The organic phases were combined and dried over anhydrous Na2SO4 overnight. The upper clear layer was then decanted and concentrated to give the title compound as white solid (5.41 g, 99%). 1H NMR (DMSO-d6) δ: 3.81 (d, J=13.4 Hz, 2H), 3.60 (br. ...
preparation 3
[0072]
tert-Butyl 1-imino-1λ4,4-thiazinane-4-carboxylate 1-oxide
[0073]Trifluoroacetamide (5.82 g, 2 eq), magnesium oxide (4.05 g, 4 eq), and rhodium(II) acetate dimer (330 mg, 0.03 eq) were placed in a 250 mL round bottom flask. Dichloromethane (70 mL) under a nitrogen atmosphere was then added with stirring, followed by the addition of tert-butyl thiomorpholine-4-carboxylate 1-oxide (5.41 g, 1 eq) and diacetoxyiodobenzene (12.1 g, 1.5 eq). The reaction mixture was stirred at RT overnight. Then it was filtered through a pad of celite and silica gel, washed with DCM first then with EtOAc. The filtrate was concentrated and the resulting oily residue was taken up in MeOH (250 mL), to which was added potassium carbonate (17.3 g, 5 eq). The reaction mixture was stirred at RT for 2 hours and filtered through a pad of celite and silica gel and washed with MeOH. The filtrate was concentrated under reduced pressure and the resulting lightly brown oily residue was treated with EtOAc with stirr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
| catalytic | aaaaa | aaaaa |
| binding affinities | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com