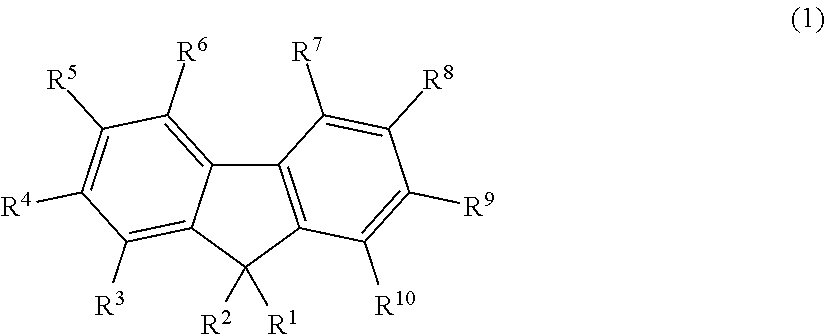
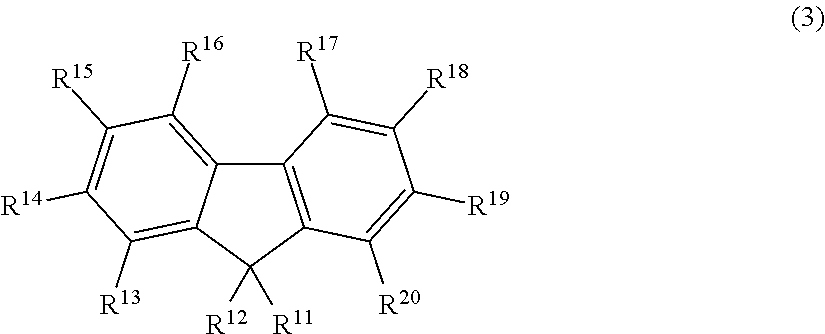
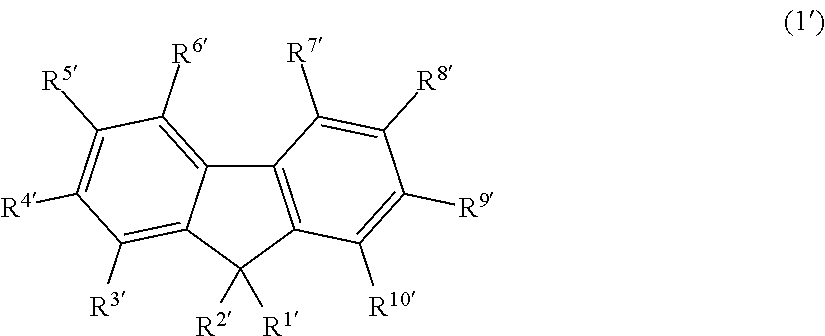
Fluorescent compound, making method, and fluorescent resin composition
a technology of fluorescent compounds and resins, applied in the field of fluorescent compounds, can solve the problems of difficult failure to improve the solubility of low polar solvents and resins such as aliphatic hydrocarbons and silicone resins, and difficulty in uniform dispersion of fluorescent compounds within resins, etc., to achieve improved compatibility with organic solvents and resins, improved purity, and easy formulation of lightness and color saturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2,7-dibromo-9,9′-di-[3-(1-trimethylsiloxy-1,3,3,3-tetramethyldisiloxan-1-yl)propyl]fluorene (Compound #1)
[0092]A 100-ml three-necked flask equipped with a reflux condenser and stirrer was purged with nitrogen and charged with 845.0 mg (2.11 mmol) of 2,7-dibromo-9,9′-diallylfluorene, 15.2 mg of a 2 wt % xylene solution of platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex, and 5 ml of dry toluene. To the flask, 1.01 g (4.54 mmol) of 1,1,1,3,5,5,5-heptamethyltrisiloxane was added dropwise over 10 minutes. After the completion of dropwise addition, the reaction solution was stirred for 6 hours at room temperature. The solution was concentrated in vacuum. Water and toluene were added to the concentrate, followed by separatory operation to extract the organic layer. The resulting solution was dried over magnesium sulfate and concentrated under reduced pressure on a rotary evaporator, yielding 1.70 g of a pale yellow liquid.
[0093]On time-of-flight mass spectrometry...
example 2
Synthesis of 2,7-bis(biphenyl)-9,9′-di-[3-(1-trimethyl-siloxy-1,3,3,3-tetramethyldisiloxan-1-yl)propyl]fluorene (Compound #2)
[0096]A 100-ml three-necked flask equipped with a reflux condenser and stirrer was purged with nitrogen and charged with 1.70 g (2.00 mmol) of 2,7-dibromo-9,9′-di[3-(1-trimethylsiloxy-1,3,3,3-tetramethyldisiloxan-1-yl)propyl]-fluorene, 1.02 g (5.15 mmol) of biphenylboronic acid, 131.4 mg (0.11 mmol) of tetrakisi(triphenylphosphine), and 20 ml of dimethoxyethane. To the flask, 1.10 g (7.96 mmol) of potassium carbonate in 4 ml of water was added dropwise. After the completion of dropwise addition, the reaction solution was stirred for 4.5 hours at 67° C. Water and toluene were added to the solution, followed by separatory operation to extract the organic layer. The resulting solution was dried over magnesium sulfate, concentrated under reduced pressure on a rotary evaporator, and purified by HPLC, yielding 1.48 g of a white solid.
[0097]On NMR and MALDI-TOFMS spe...
example 3
Synthesis of 2,7-dibromo-9,9′-di-[3-(undecamethylpenta-siloxan-1-yl)propyl]fluorene (Compound #3)
[0102]A 100-ml three-necked flask equipped with a reflux condenser and stirrer was purged with nitrogen and charged with 1.33 g (3.29 mmol) of 2,7-dibromo-9,9′-diallylfluorene, 15.0 mg of a 2 wt % xylene solution of platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex, and 6 ml of dry toluene. To the flask, 3.00 g (8.09 mmol) of 1,1,3,3,5,5,7,7,9,9,9-undecamethyltrisiloxane was added dropwise over 10 minutes. After the completion of dropwise addition, the reaction solution was stirred for 7 hours at room temperature. Water and toluene were added to the solution, followed by separatory operation to extract the organic layer. The resulting solution was dried over magnesium sulfate, concentrated under reduced pressure on a rotary evaporator, and purified by silica gel chromatography, yielding 2.63 g of a pale yellow liquid.
[0103]On MALDI-TOFMS analysis, the liquid was identified to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| fluorescent | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| transparency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com