Methods and pharmaceutical compositions for the treatment of respiratory tract infections
a technology of respiratory tract infections and pharmaceutical compositions, applied in the direction of antibacterial agents, aerosol delivery, peptide/protein ingredients, etc., can solve the problems of irritability, poor appetite, runny or stuffy nose,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
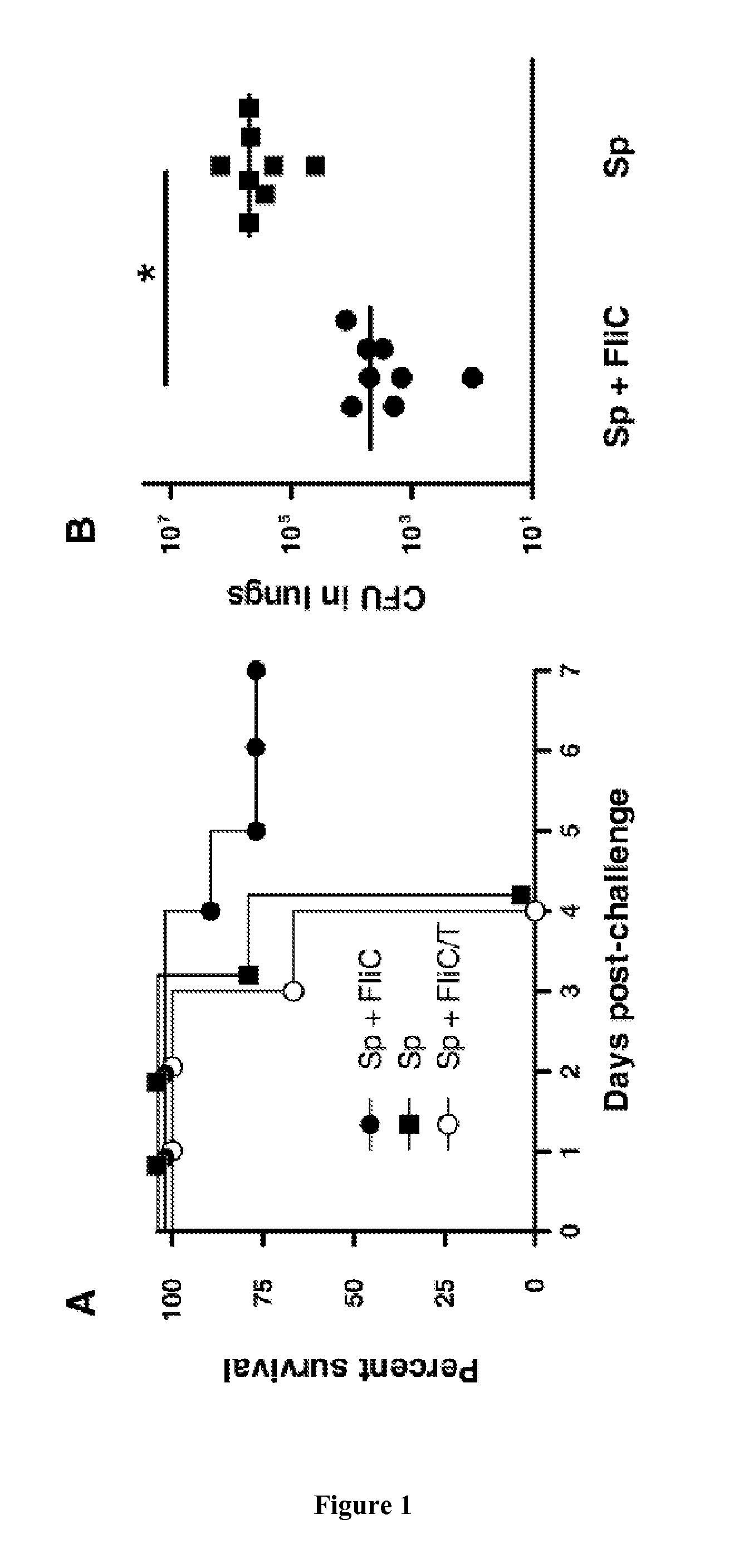
[0078]Material & Methods
[0079]Bacterial Preparation:
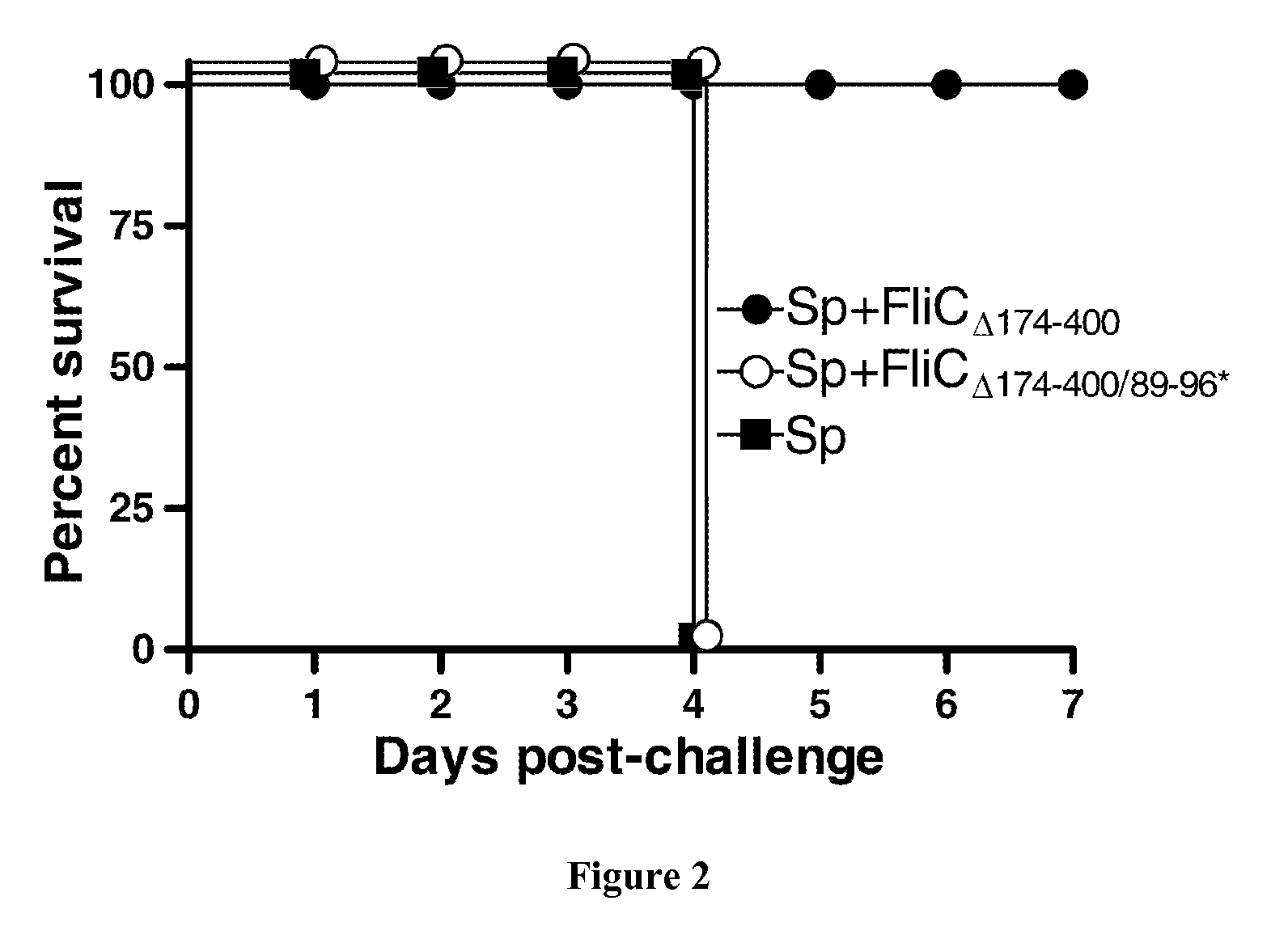
[0080]Streptococcus pneumoniae serotype 1 (clinical isolate E1586) was obtained from the National Reference Laboratory—Ministry of Health, Uruguay (39). Working stocks were prepared as follows. Todd Hewitt Yeast Broth (THYB) (Sigma-Aldrich, St. Louis—Mo., USA) was inoculated with fresh colonies of S. pneumoniae grown in blood-agar plates, and incubated at 37° C. until the culture reached OD600nm of 0.7-0.9 units. Cultures were stored at −80° C. in THYB+glycerol 12% (v / v) up to 3 months. For mouse infection, working stocks were thawed and washed with sterile physiological saline solution (saline) and diluted to the appropriate concentration. Number of bacteria in stocks was confirmed by plating serial dilutions onto blood agar plates.
[0081]Proteins:
[0082]Native flagellin (FliC) from Salmonella enterica Serovar Typhimurium SIN22, or recombinant flagellins (FliCΔ174-400 and FliCΔ174-400 / 89-96*) were prepared as previously described (2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com