Dual Specific Immunotoxin for Brain Tumor Therapy
a brain tumor and immunotoxin technology, applied in the field of anti-tumor immunotoxins, can solve the problem of increasing the overall median survival by only 75 days
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0029]Cell Lines.
[0030]Cell lines expressing EGFRwt used were the human epidermoid carcinoma cell line A431 {Merlino, 1984} and the murine Swiss 3T3 mouse fibroblast cell line EGFRwt transfectant NR6W {Batra, 1995}. Cell lines transfected to express EGFRvIII included the murine Swiss 3T3 mouse fibroblast cell line-derived transfectant NREM {Batra, 1995}. The parental murine Swiss 3T3 mouse fibroblast cell line, NR6, was used as control. All cell lines were cultured in complete zinc option-10% fetal bovine serum (FBS) (Richter's zinc option; Invitrogen, San Diego, Calif.) and passed at confluence using 0.05% Trypsin-EDTA (Invitrogen).
[0031]Disaggregation of Xenograft Tumor Samples.
[0032]Xenograft tissues from malignant glioma (D256MG, D270MG, D2159MG) obtained under sterile conditions from the Duke animal facility were prepared for cell culture in a laminar flow hood with a sterile technique. Tumor material was finely minced with scissors and added to a trypsiniz...
example 2
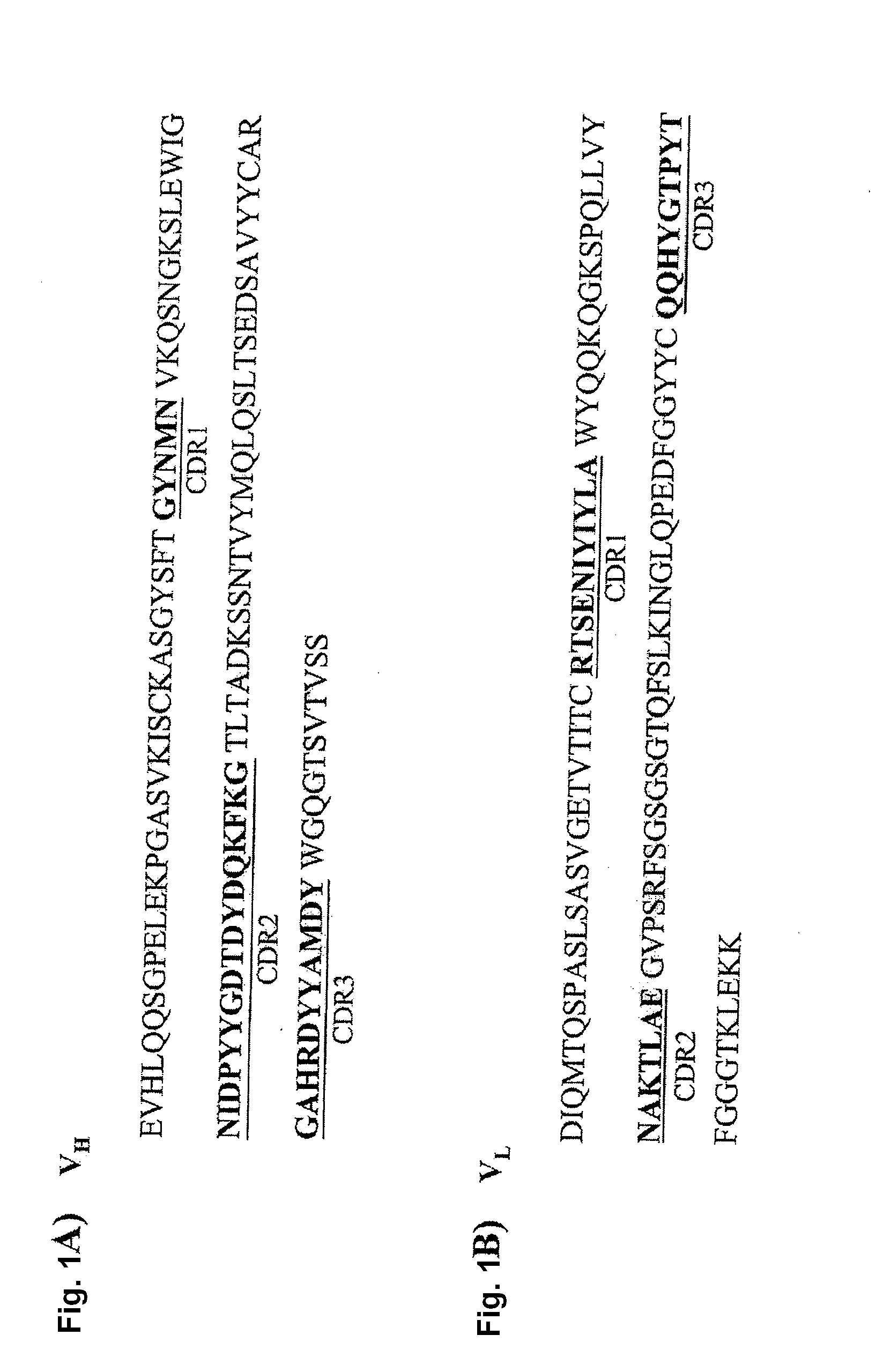
Cloning of the VH and VL Domain of D2C7IgG1κ
[0050]VH and VL cDNAs were isolated from the D2C7 hybridoma by a RACE method as described in “Materials and Methods.” The heavy-chain and light-chain variable domains were cloned and sequenced. The amplified VH and VL fragments were approximately 360 and 321 bp, respectively. The deduced amino acid sequences of the D2C7 VH and VL domains are shown in FIG. 1. Sequence analysis of the VH and VL amino acids, using the database of germ-line genes (http: / / www.ncbi.nlm.nih.gov / igblast / ), revealed that the sequences were derived from different germ-line V genes with a similarity of 70% to 75%.
example 3
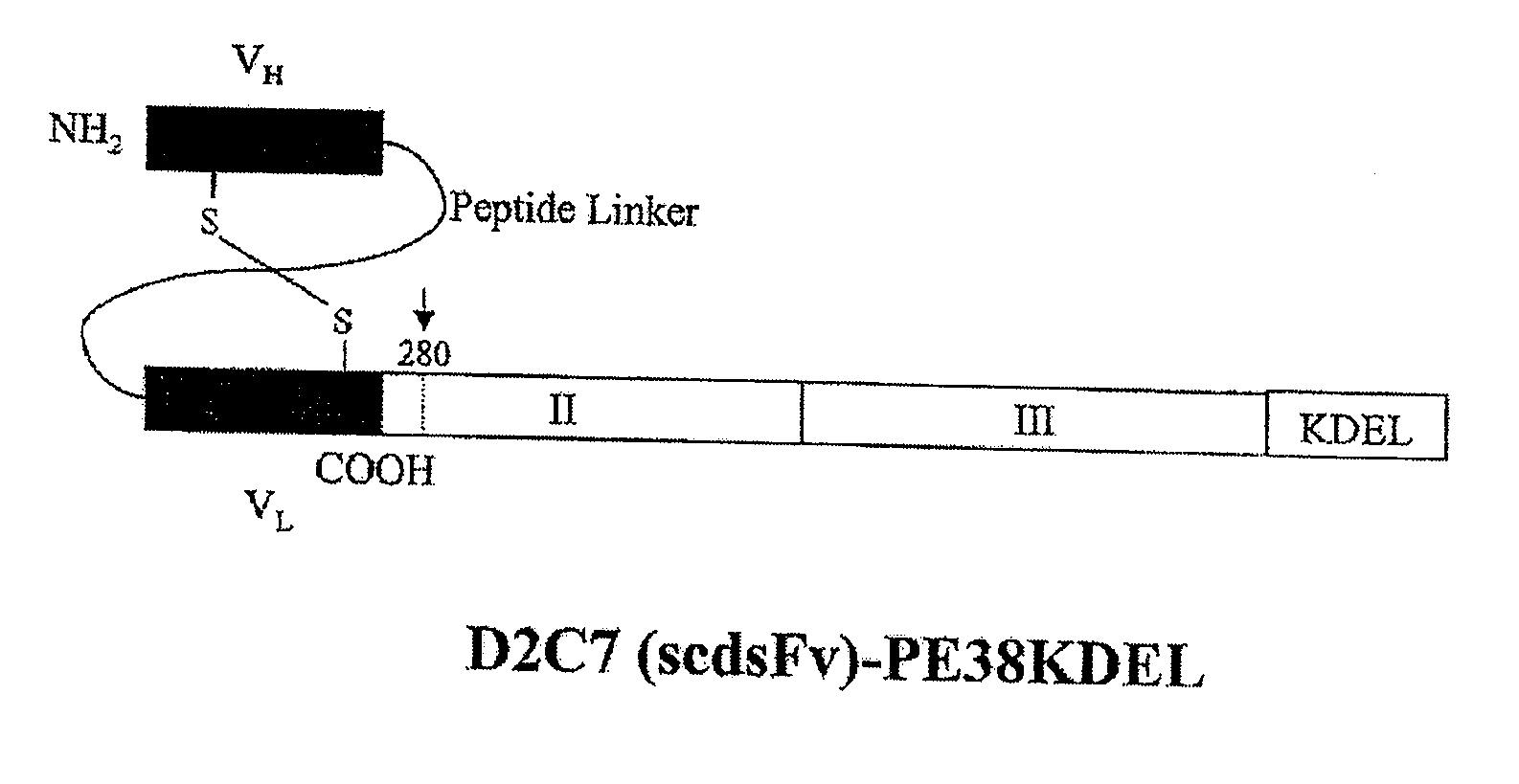
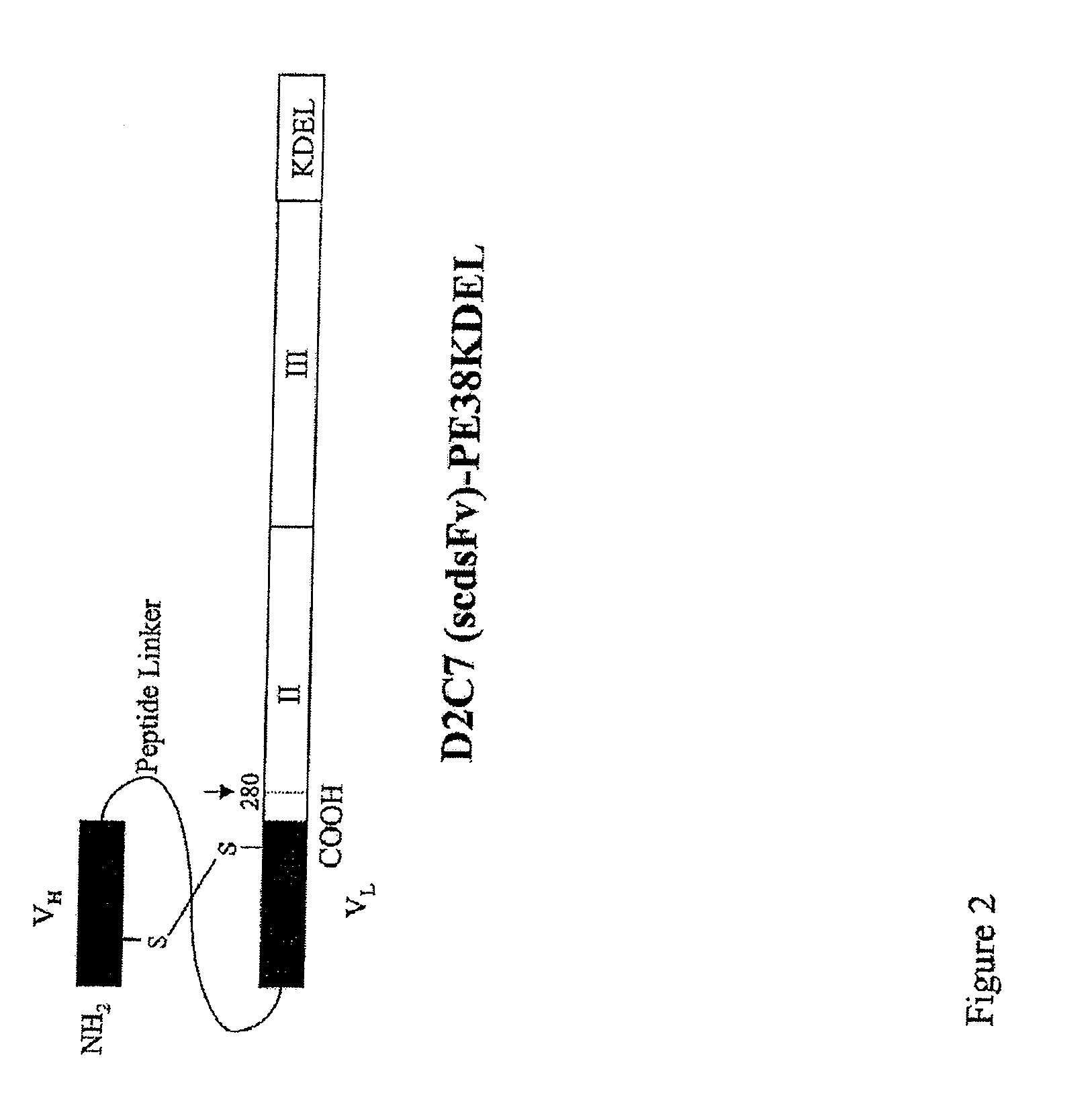
Construction, Expression, and Purification of D2C7-(scdsFv)-PE38 KDEL Immunotoxin
[0051]The carboxyl terminus of the D2C7 VH domain was connected to the amino terminus of the VL domain by a 15-amino-acid peptide (Gly4Ser)3 linker (SEQ ID NO: 11). In order to obtain a stable IT, it is essential to ensure that during renaturation VH is positioned near VL. This was achieved by mutating a single key residue in each chain to cysteine, for the stabilizing disulfide bond to form. On the basis of predictions using molecular modeling and empirical data with other dsFv-recombinant ITs, we chose one amino acid in each chain to mutate to cysteine {Reiter, 1996}. These are residues 44 in the framework region 2 (FR2) of VH and 100 in the FR4 of VL (according to the Kabat numbering). Thus, we prepared an Fv that contains both a peptide linker and a disulfide bond generated by cysteine residues that replace Ser44 of VH and Gly100 of VL. The D2C7 (scdsFv) PCR fragment was then fused to DNA for domain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com