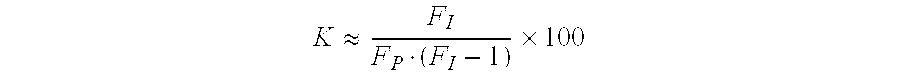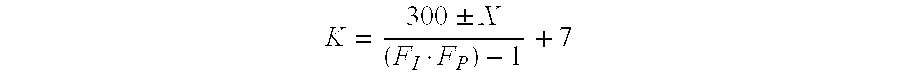Infection-resistant polyurethane foams, method for producing the same and use thereof in antiseptic wound dressing
a polyurethane foam and infection-resistant technology, applied in the direction of disinfectants, biocide, bandages, etc., can solve the problems of limited release of micelle therapeutic ingredients, limited bioavailability, and large or chronic wounds are particularly prone to infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
Example 1
[0100]In a paperboard cup, 64.22 g of Levagel® SN 100 [Bayer MaterialScience AG, polyol interpolymer of propylene oxide and ethylene oxide (EO), pentaerythritol starter, ethylene oxide end block, OH number 4, average molecular weight 6400, EO content 20% by weight, stabilized with 0.5% by weight of 2,6-di-tert-butyl-4-methylphenol (BHT)] are admixed with 27.52 g of FAVOR®-PAC 230 (Degussa AG, superabsorbent based on polyacrylic acid, salt of a crosslinked grafted polyacrylic acid-polyalcohol copolymer) and 1.00 g of PHMB hydrochloride, previously supplementarily dried at 80° C. in a vacuum drying cabinet, by stirring. This is followed by addition of a freshly prepared solution of 0.055 g of dibutyltin laurate in 2.70 g of Levagel® SN 100 and thorough commixing. Lastly, 5.505 g of Desmodur® E 305 (Bayer MaterialScience AG, NCO-terminated prepolymer based on 7 mol of hexamethylene diisocyanate and 1 mol of polypropylene oxide and having an average molecular weight of 400 g / mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| pore sizes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com