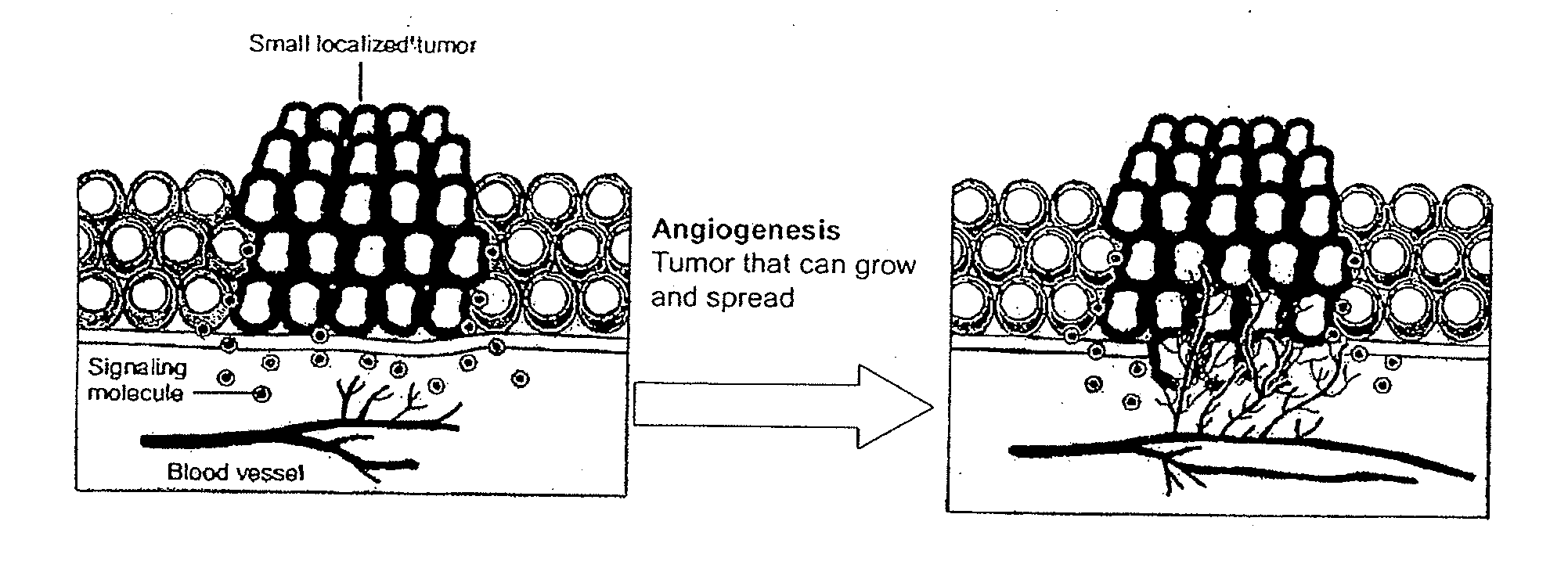
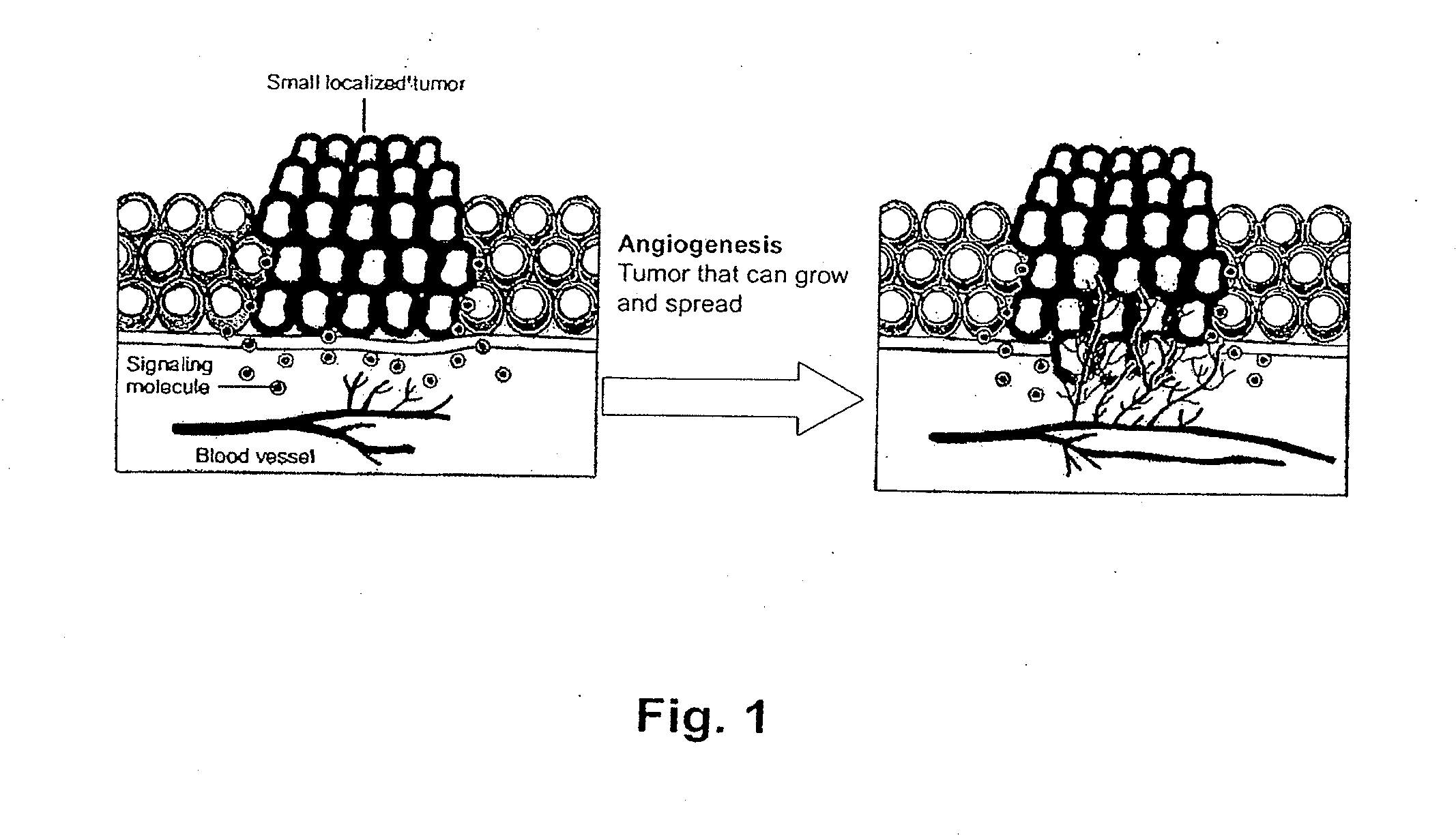
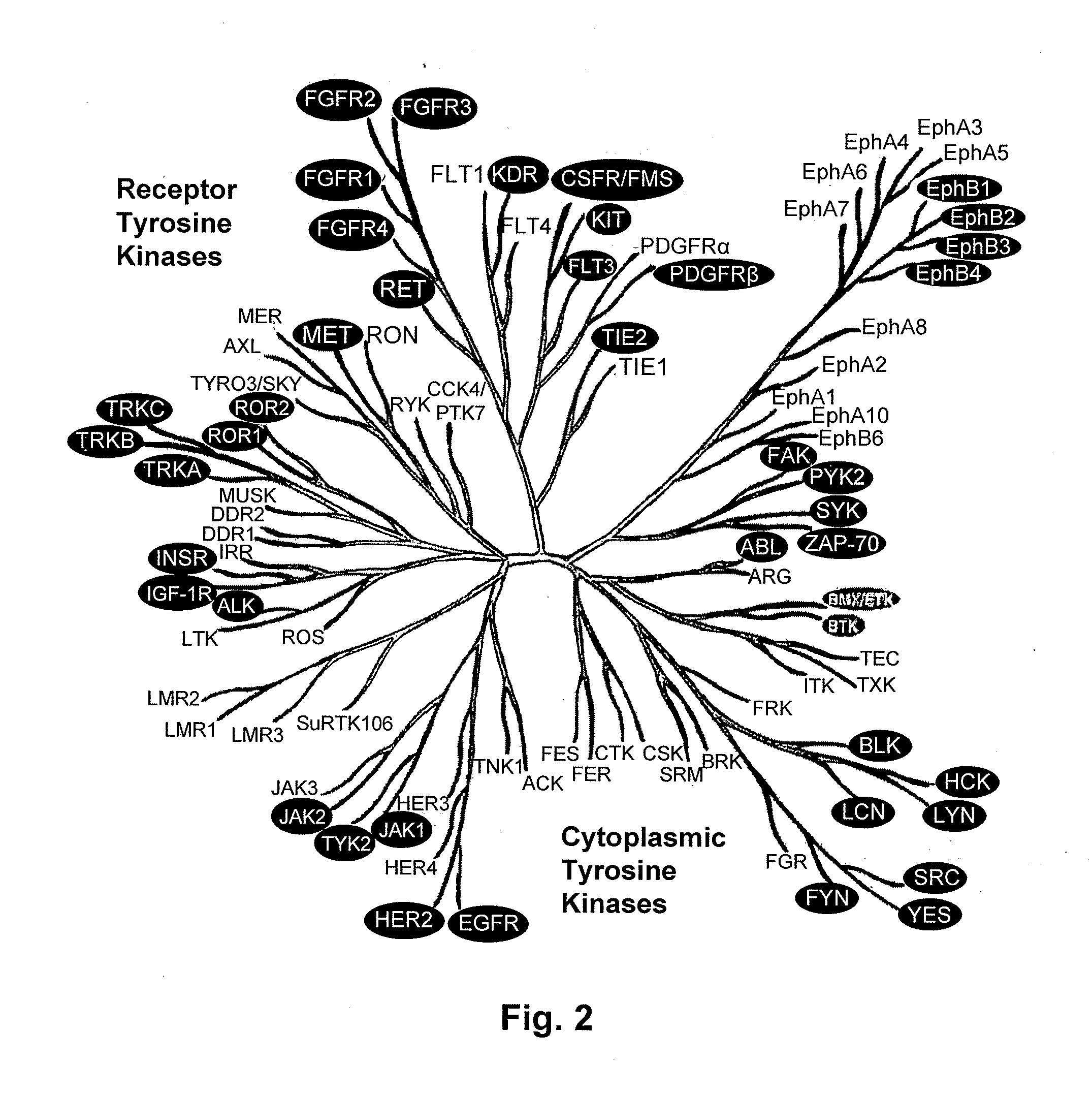
Detection of Activation of Endothelial Cells as Surrogate Marker for Angiogenesis
a technology of endothelial cells and surrogate markers, which is applied in the direction of instruments, biological materials, material analysis, etc., can solve the problems of no quantitative method for determining the total angiogenic output of a patient's tumor burden, bleeding and blindness, tumor regression and dormancy, etc., and achieves the effect of sensitive and convenient measurement of angiogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Immunomagnetic Isolation of Human Endothelial Cells
[0283]Substantially pure CEC and CECP can be isolated by using the immunomagnetic isolation / enrichment technique described in Kinzler et al. (2000) Science 289:1197-1202. Briefly, the epithelial and hematopoietic cell fractions in the peripheral blood samples are sequentially removed via negative selection via antibody-linked magnetic beads (BerEP4 beads—Epithelial, CD45 beads—Pan leukocyte, CD64 beads—Macrophages, and CD14 beads—Monocytes). The remaining cells are stained with P1H12 antibodies and are isolated via positive selection with magnetic beads.
[0284]In this example, CEC and CECP were isolated by using a protocol modified from Voest et al. (2004) Annals of Oncology 15: 139-145 as follows:[0285]1) Magnetic beads (Dynal M450 IgG1, Dynal AS, Oslo, Norway) were conjugated to a monoclonal antibody for CD146, MCAM, an endothelial cell surface junction protein.[0286]2) Whole blood was diluted 1:3 with 0.9% NaCl and incubated for 3...
example 2
Lysis of CEC or CECP
[0313]The isolated CEC or CECP were lysed according to the following protocol:[0314]Protocol[0315]Step[0316]1) Seed 2 15 cm dishes with 6×10̂6 cells in 20 mL EGM[0317]2) Prepare fresh lysis buffer[0318]3) Starting now, always work on ice. Aspirate the medium.[0319]4) Add 600 ul of fresh lysis buffer.[0320]5) Swirl the plates to distribute the buffer evenly.[0321]6) Scrap the cells.[0322]7) Collect the crude lysate in 1.5-ml tube.[0323]8) Wait for 30 min.[0324]9) Spin down at 4 C at 14000 rpm for 10 min[0325]10) Collect the supernatant in 1.5-ml new tube.[0326]11) Store at −80 C for later use.[0327]The lysis buffer was prepared by mixing the following reagents:
LysisStock10 mlbufferReagentscon.Final concVol1)10% triton X-1001011.002)1M Tris pH 7.510.050.503)1M NaF10.050.504)5M NaCl50.10.205)2M B-Glycerol-10.050.50phosphate6)0.1M Na3VO40.10.0010.107)1 mg / ml pepstatin10.010.108)Complete mini1 tabletprotease9)0.5M EDTA0.50.0050.10Total (ml)3.00Water (ml)7.00
example 3
Analysis of Cell Lysate for Protein Complexes of Angiogenic Receptors and Phosphorylation of Downstream Effector Proteins
[0328]Protein complexes formed by angiogenic receptors (e.g., VEGFR, Tie), such as the homodimers and heterodimers formed by VEGFR, were measured in cell lysates from CEC, CECP, and cell lines such as human umbilical vein endothelial cells (HUVEC), and human vulval carcinoma cell line (A431). Measurements were made using three binding compounds and a cleaving probe by using the following protocol.[0329]1) Add Lysis Buffer and Lysate into 96-well PCR plate to 30 uL final volume.[0330]2) Add 5 uL antibody mix to each well.[0331]3) Incubate reactions at RT for 2 hours on plate shaker.[0332]4) Add 5 uL steptavidin-scissor mix to each well in dark room.[0333]Mix is 32.5×. Added 15 uL to 472.5 uL Dilution Buffer (1% BSA in PBS)[0334]5) Incubate reactions at RT for 45 minutes on plate shaker in dark room.[0335]6) Pre-wet 96-well 2 micron membrane filter plates with 100 u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com