Semen analysis
a technology of semen and sex, applied in the field of semen analysis, can solve the problems of large statistical errors, high work intensity, and high labor intensity of manual assessment, and achieve the effect of adding confidence to the processing results and high quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0080]As stated above, the automatic optical measurement of TSC in human semen samples as opposed to animal samples has been hampered in the past due to the low concentration of sperm cells. This, together with the high background electronic and optical noise due e.g. to seminal plasma variability has prevented the application of methods routinely used in veterinary fertility analysis. The method of the present invention comes to overcome these obstacles by combining the following features:[0081](i) the sample is placed in a transparent container between a synchronically pulsed light source and a synchronically enabled photodetector. The use of a synchronically pulsed light source and photodetector enables the distinction of sperm cells at low concentrations over electronic noise levels.[0082](ii) measuring the optical absorbance of the sample in the range of 800-1000 nm. It has been found that measuring the absorbance in the near infrared region provides the optimal conditions for ...
example 2
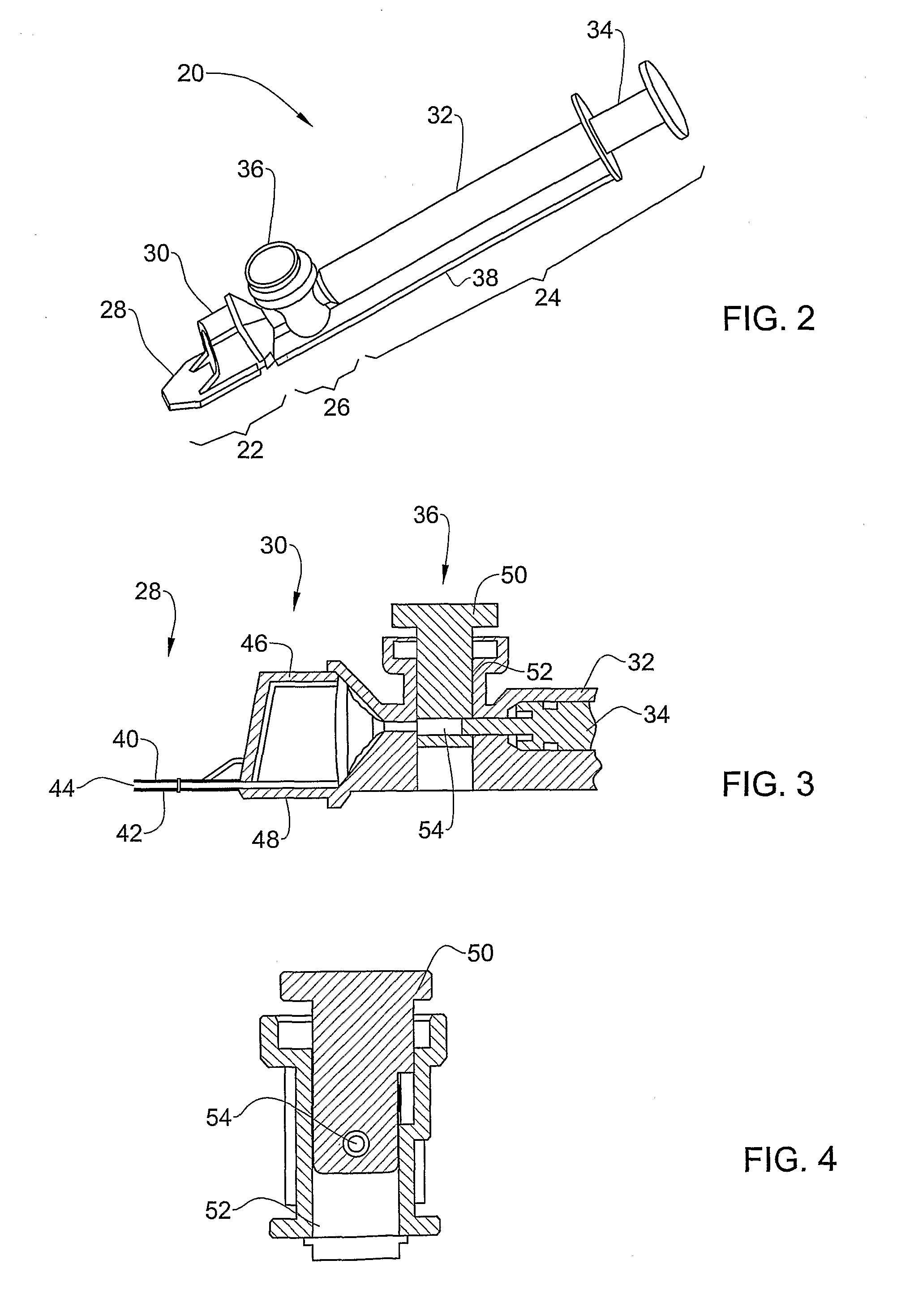
[0087]FIG. 2 illustrates one embodiment of a sampling device 20 according to the invention, for use in measuring semen. The device comprises an anterior optical viewing section 22, a posterior aspirating section 24 and an intermediate air exclusion section 26.
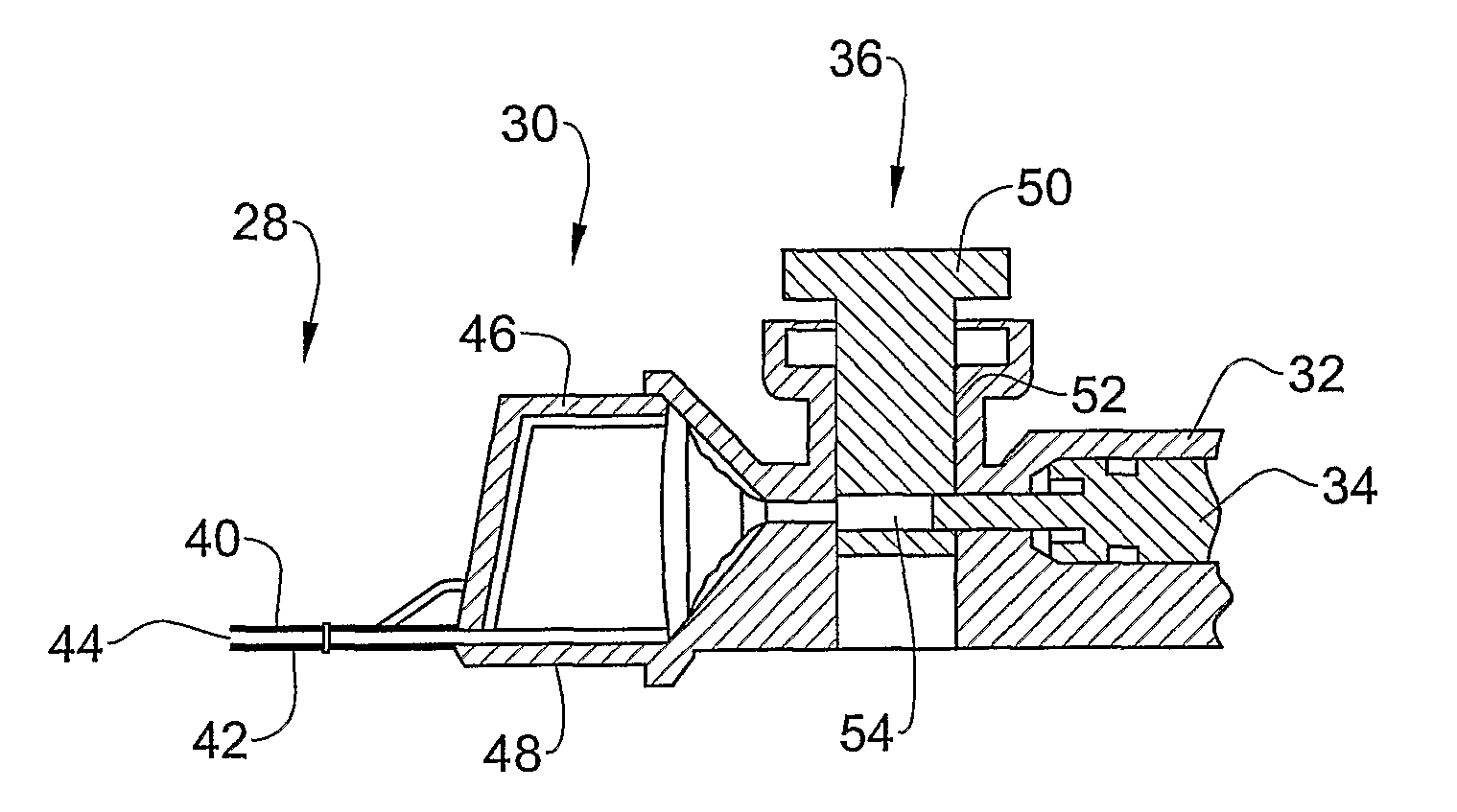
[0088]The optical viewing section 22 comprises a thin measuring chamber 28 and a thick measuring chamber 30. The thin chamber is used to measure MSC and / or for visualization, while the thick chamber is used to measure TSC. In this way, multiple parameters can be measured simultaneously using the same sampling device and sampling step.
[0089]The aspirating section 24 comprises a cylinder 32 and a plunger 34 slidingly inserted therein. These parts match each other and function as in a standard syringe. This section serves for the aspiration of the semen sample into the measuring chambers
[0090]The air exclusion section 26 comprises a separating valve 36 for separation of the measuring chambers from the cylinder volume after filling...
example 3
[0101]As mentioned above, determination of the MSC according to the invention requires the generation of a voltage signal which is proportional to the MSC. FIG. 5 shows one embodiment of a system for semen analysis capable of generating such a signal.
[0102]An optical capillary 100 having a rectangular cross-section is used to hold a semen sample 102. The capillary 100 is illuminated with an incident light beam 105 produced by a light source 110. The capillary 100 has an optical path of 300 μm through which the light beam 105 passes. After passing through the capillary, the scattered beam 106 is collimated by a round aperture 108 having a diameter of 70 μm. The collimated beam 107 impinges upon a photodetector 115. The photodetector 115 produces an analog voltage signal 120 proportional to the intensity of the beam 107. The analog signal varies in time due to the motility of the sperm in the semen sample 102, as shown for example in FIG. 8. The analog signal 120 is inputted to an ana...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com