Methods and compositions for seamless cloning of nucleic acid molecules
a technology of nucleic acid molecules and compositions, applied in the field of biotechnology and molecular biology, can solve the problems of affecting the structure and function of desired gene products, relying on restriction sites, and amplification products that contain extraneous polynucleotides,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of Interfering RNA Using a Seamless Cloning Vector
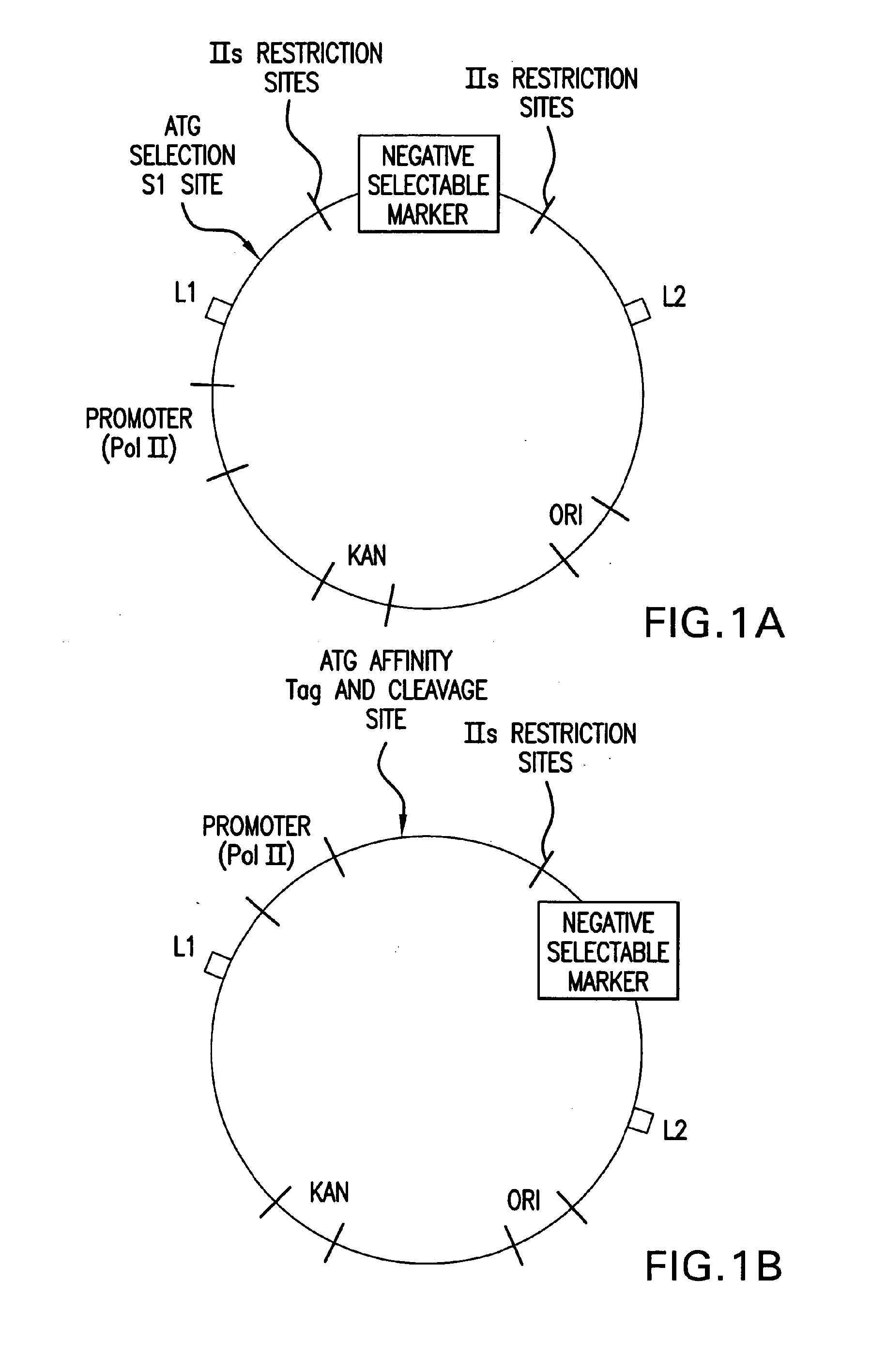
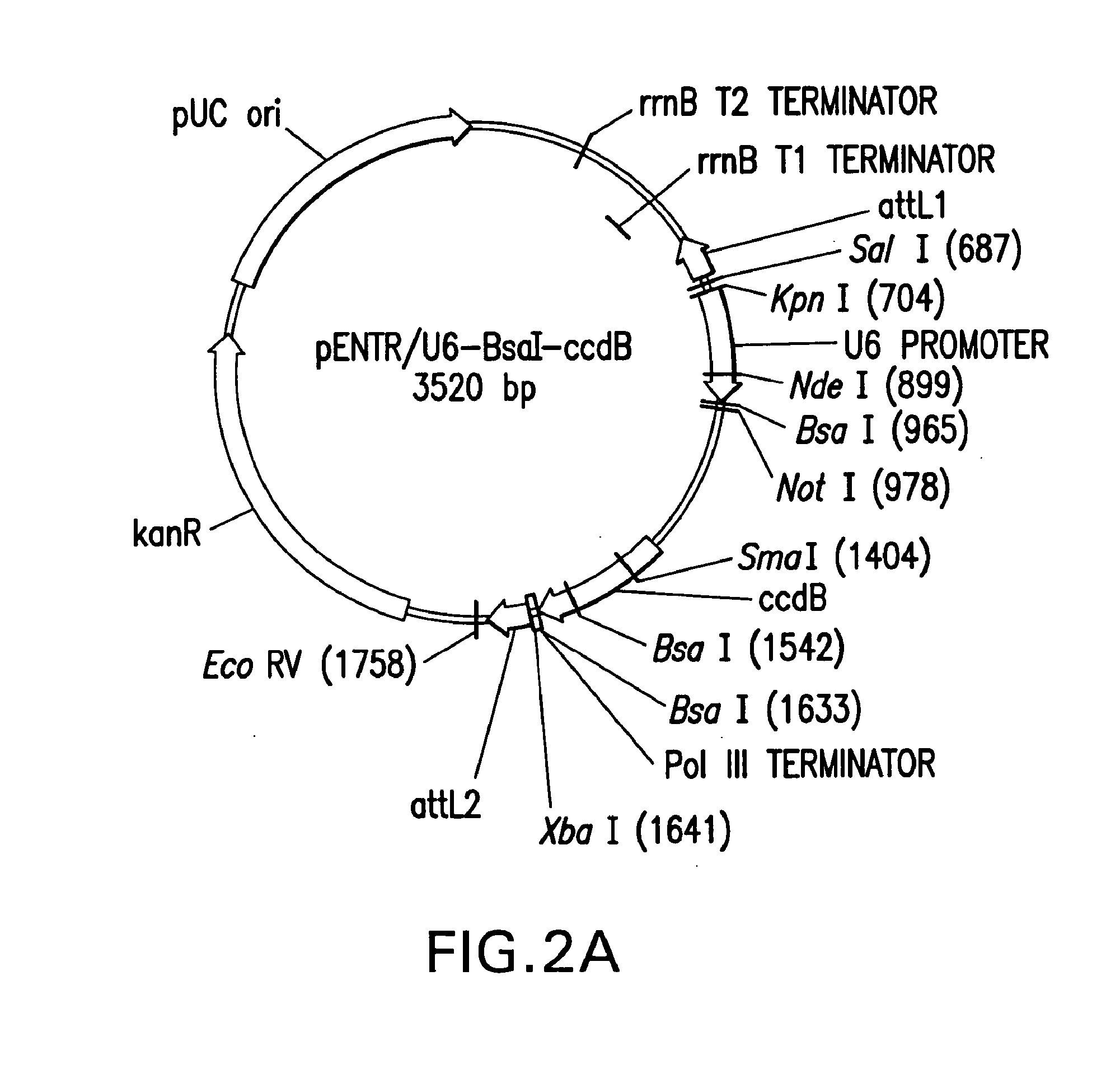
[0248]The expression of short interfering hairpin RNA molecules (shRNA) in vivo can decrease the expression of genes with complementary sequences by RNA interference (RNAi) as described previously. The seamless cloning vector described here (pENTR / U6) allows for rapid and efficient cloning of double-stranded oligonucleotide pairs (˜47 bp) coding for a desired shRNA target sequence into a Pol III U6 expression cassette. The resulting shRNA vector contains an RNAi cassette flanked by attL sites. Therefore, the pENTR / U6 shRNA vectors can be used directly for transient transfection to test various shRNA target sequences, as well as to transfer the best shRNA cassettes to Lenti and Adenoviral DEST vectors for delivery into “hard to transfect” cells.
Kit Components.
[0249]Purified, BsaI-linearized pENTR / U6.2 (once it is cut with BsaI, i.e. the linear vector is called pENTR / U6) (Catalog No. K4945-00 and K4944-00, Invitrogen, Corp.,...
example 2
Expression of Interfering RNA Using a Seamless Cloning Vector
Abstract and Introduction
[0290]Short hairpin RNA (shRNA) expression cassettes built into the U6 RNAi Entry Vector can be used to transiently knockdown genes of interest in cell culture. However, the Entry Vector carries no marker for selection in mammalian cells, and the plasmids must be introduced into cells by transfection. Transfection efficiency varies widely between cell lines and is ineffective in primary and terminally differentiated cells. In contrast to plasmid transfection, lentiviral delivery allows simple, stable transduction of a wide variety of cell types including primary and terminally differentiated cells. A number of recent publications describe the use of lentiviruses to deliver shRNAs to mammalian cells (Abbas-Terki et al. 2002, Dirac & Bernards 2003, Matta et al. 2003, Qin et al 2003, Rubinson et al 2003, Stewart et al 2003, Tiscornia et al. 2003), demonstrating an existing interest in this technique.
[...
example 3
RNAi Using Block-iT™ Dicer Kit
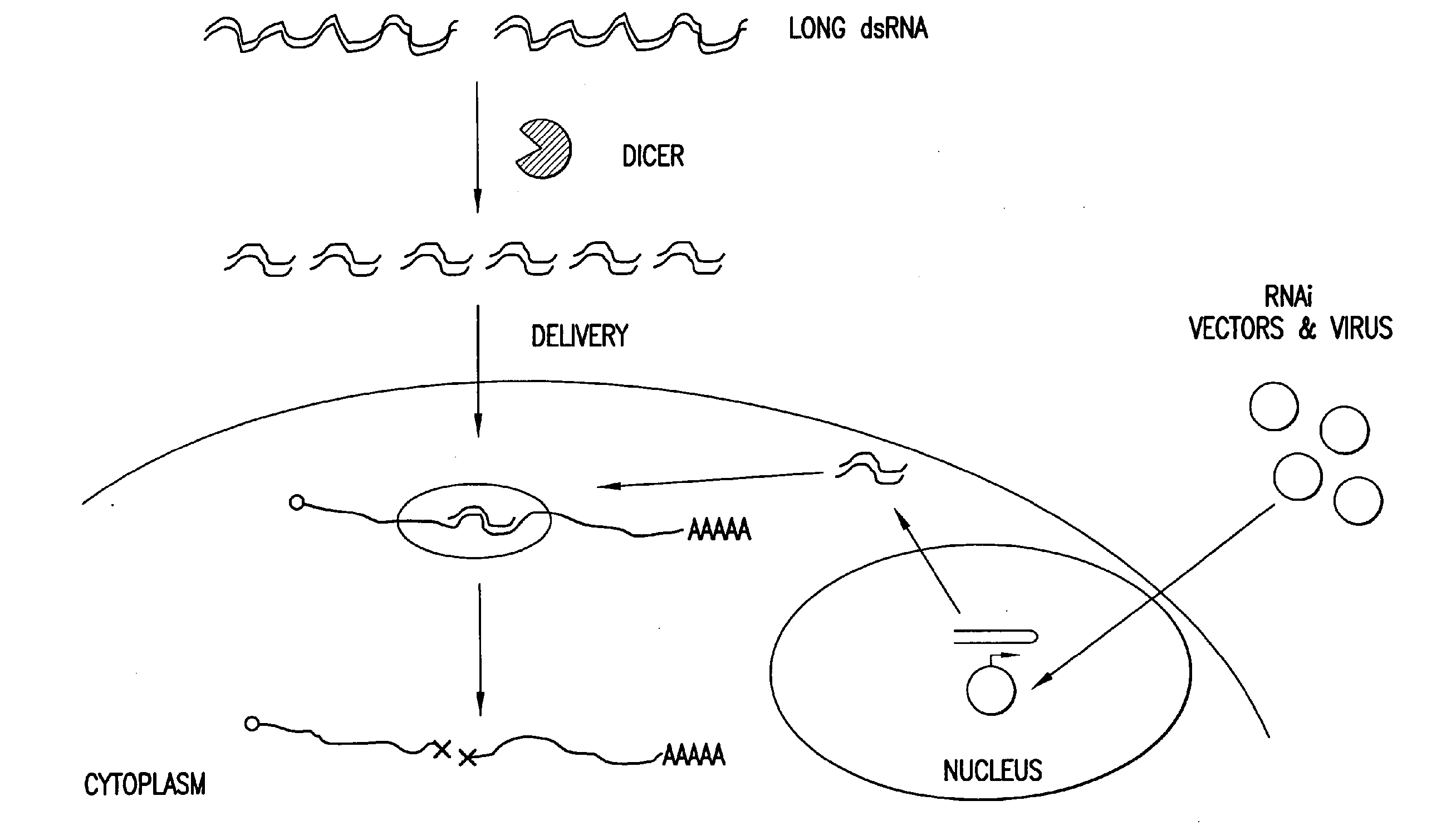
[0306]BLOCK-iT™ Kits (Invitrogen Corporation; Carlsbad, Calif.) can be used for fast and efficient RNAi applications. Eukaryotic cells naturally regulate gene expression with dsRNA. A BLOCK-iT™ Dicer Kit can be used to generate dsRNA that are then diced into siRNA, purified and transfected into cells. The BLOCK-iT™ Dicer Kit requires no expensive synthetic siRNAs. It also produces a pool of many siRNAs per gene, not just one or a few, which means a higher probability of knockdown (FIGS. 21, 22, and 23). A purification procedure gives a high yield of siRNAs in a transfection-ready buffer and virtually eliminates remaining long dsRNA and cleave intermediates.
[0307]BLOCK-iT™ Long RNAi Transcription Kits use a T7 TOPO linker which allows any polymerase chain reaction (PCR) product to become a template for transcription (FIG. 20). This mediates RNAi in invertebrates (e.g., insects, nematodes and protozoans), some mammalian embryonic cells (undifferentiated E...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
| Recombination enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com