Three Dimensional Cell Protector/Pore Architecture Formation for Bone and Tissue Constructs
a cell protector and pore structure technology, applied in the field of three-dimensional cell protector/pore architecture formation of bone and tissue constructs, can solve the problems of no cells being mixed into the cpc paste, no prior art design of cell protector/pore architecture, etc., and achieve the effect of protecting from mechanical and chemical damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0046]An example of manufacture of this invention would be for medical, veterinary, dental, craniofacial and orthopedic bone repair. The CPC powder and liquid are mixed to form a paste that is filled into a stainless steel mold of 6 mm diameter and 3 mm depth. Distilled water is used as the cement liquid. Each specimen in the mold is sandwiched between two glass slides, and the assembly is incubated in a humidor with 100% relative humidity at 37° C. The hardening time is measured using a needle method with a load of 453.5 g and a flat tip diameter of 1.06 mm. A cement specimen is considered set when the needle loaded onto the specimen surface failed to leave a perceptible indentation. The time measured from the paste being mixed to this point is used as the setting time. Depending on composition and powder-to-water ratio, the setting time ranges from (14.5±1.3) min (82.8±4.4) min.
[0047]When the liquid contains water with sodium phosphate and hydroxypropyl methylcellulose, the cement...
example 2
[0048]An example of manufacture of this invention would be to incorporate living cells into a calcium phosphate cement for tissue repair. As an example, MC3T3-E1 osteoblast-like cells (Riken, Hirosaka, Japan) are cultured following established protocols (Simon et al., Preliminary report on the biocompatibility of a moldable, resorbable, composite bone graft consisting of calcium phosphate cement and poly(lactide-co-glycolide) microspheres. J. Orthop Res 20:473-482, 2002; Xu et al., Self-hardening calcium phosphate composite scaffold for bone tissue engineering. Journal of Orthopaedic Research 22:535-543, 2004). Cells are cultured in flasks at 37° C. and 100% humidity with 5% CO2 (volume fraction) in a. modified Eagle's minimum essential medium (Biowhittaker, Walkersville, Md.). The medium is supplemented with 10% volume fraction of fetal bovine serum (Gibco, Rockville, Md.) and kanamycin sulfate (Sigma, St. Louis, Mo.), and changed twice weekly. Fifty thousand cells diluted into 2 m...
example 3
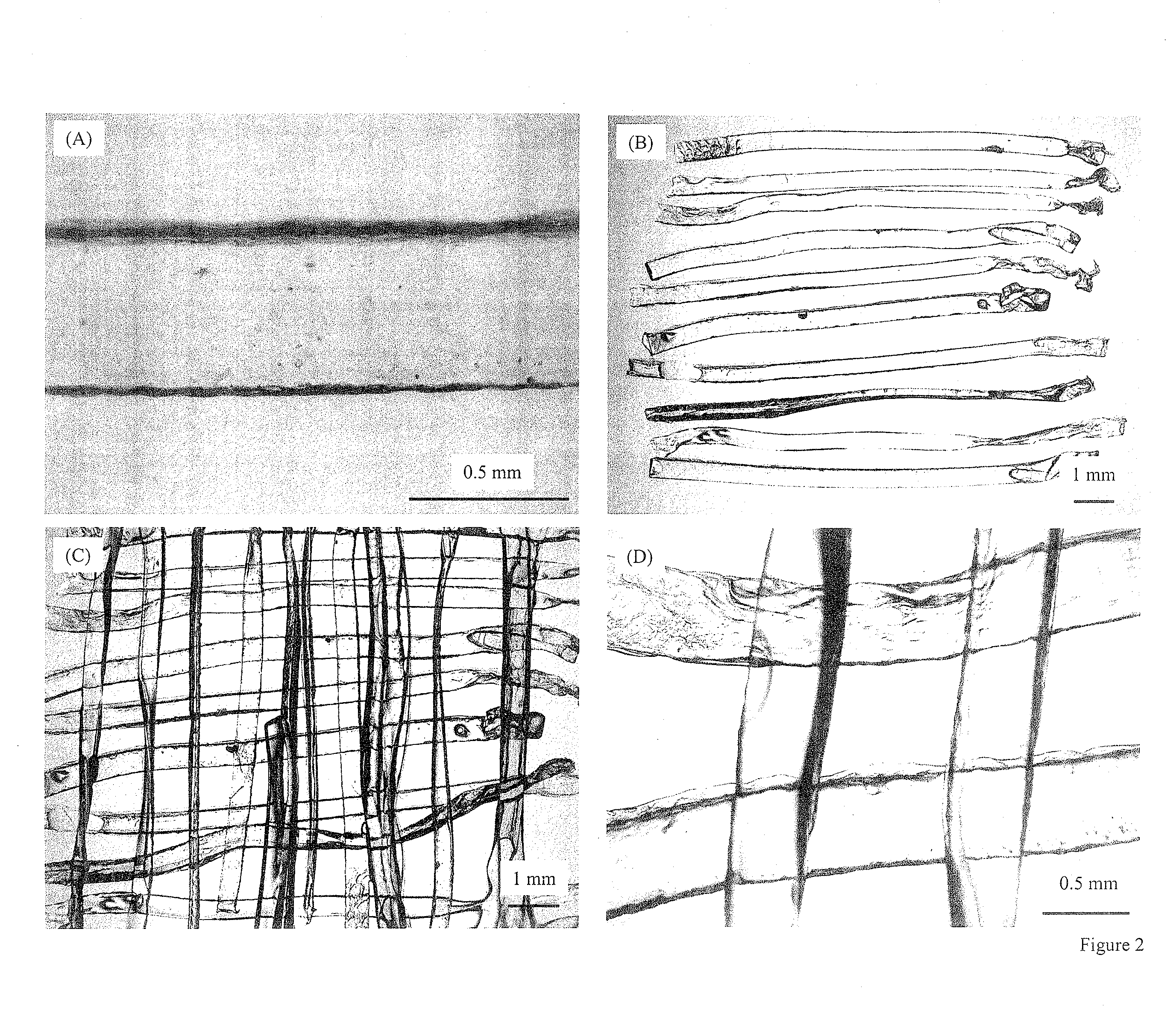
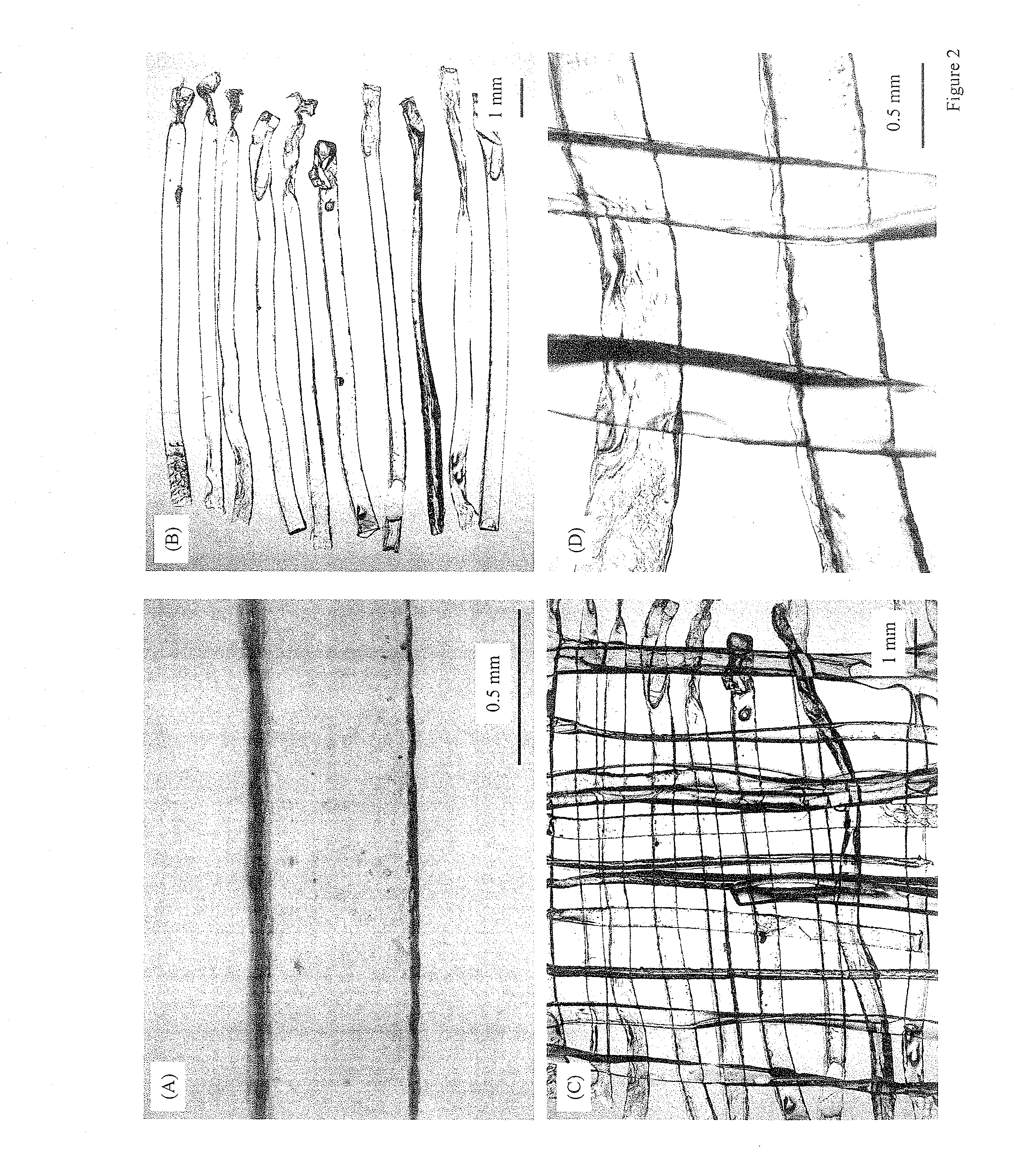
[0049]Another example of manufacture would be to produce in-situ setting CPC bone graft materials with living cells inside cell protectors that are mixed into the CPC paste. Biocompatible and degradable polymers, ceramics and composites can be used to construct the cell protectors. For example, a degradable polymer, poly(ethylene glycol)-anhydride dimethacrylate, is used as a rapidly degradable polymer for fabricating cell protectors. Structures such as those shown in FIG. 2 are fabricated by mixing osteoblast cells into the monomer liquid and then polymerizing the liquid into designed shapes inside molds. In the example in FIG. 2A, there are about 580 osteoblast cells inside each rod. These cell protector structures with living cells inside are mixed into the CPC paste, which is then mixed with an appropriate liquid and allowed to set to form an implant. The construct is immersed in water or in a physiological solution for the CPC to mature and for the cell protector to dissolve. I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com