Dermal Delivery Device
a delivery device and skin technology, applied in the direction of bandages, biocide, drug compositions, etc., can solve the problems of inadequate adhesion and/or skin irritation, and the device intended for extended wear, i.e. 3 or more days, is susceptible to inadequate adhesion and/or skin irritation, and the effect of permeation enhancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fabrication of Transdermal Drug Delivery System
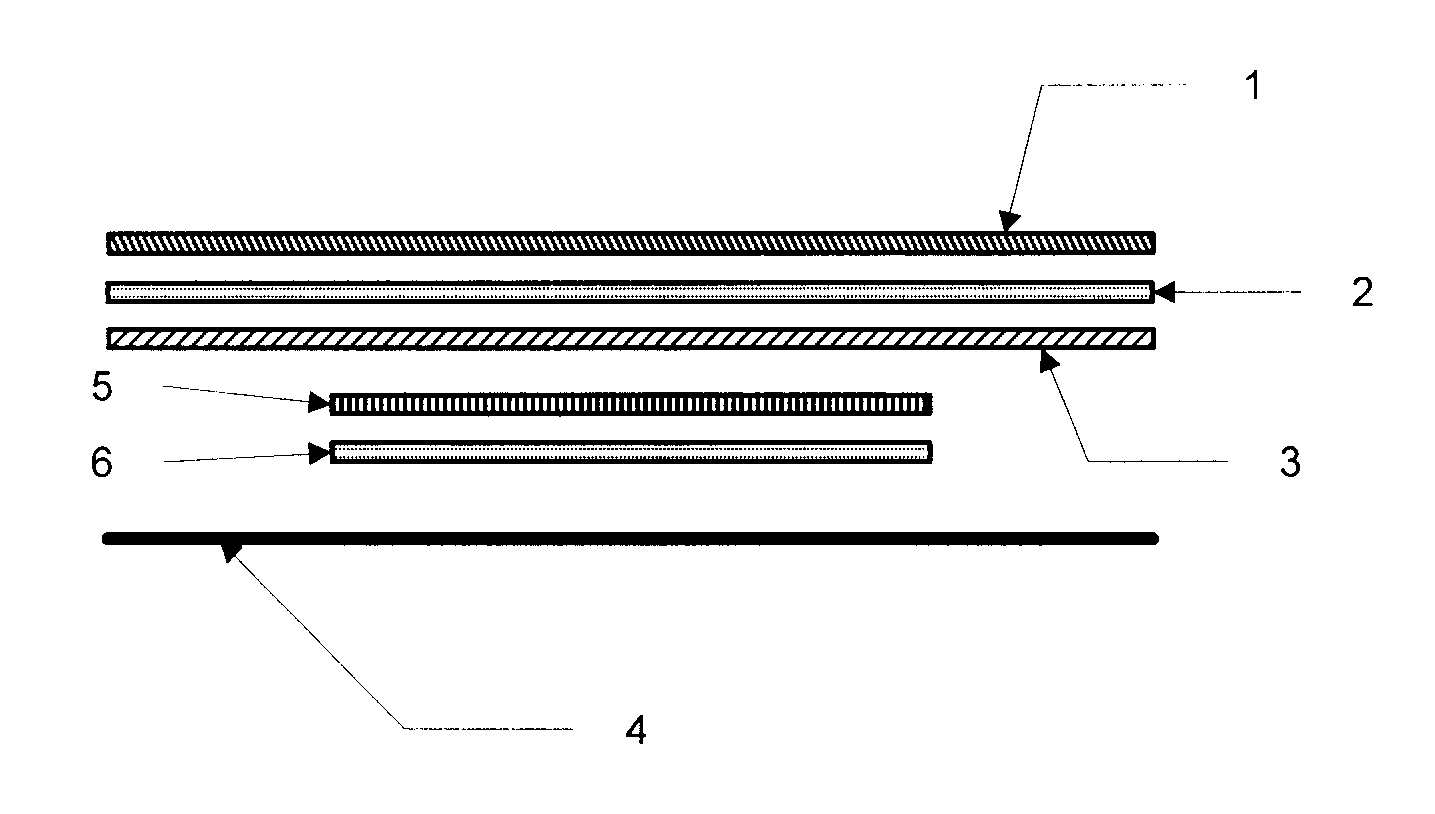
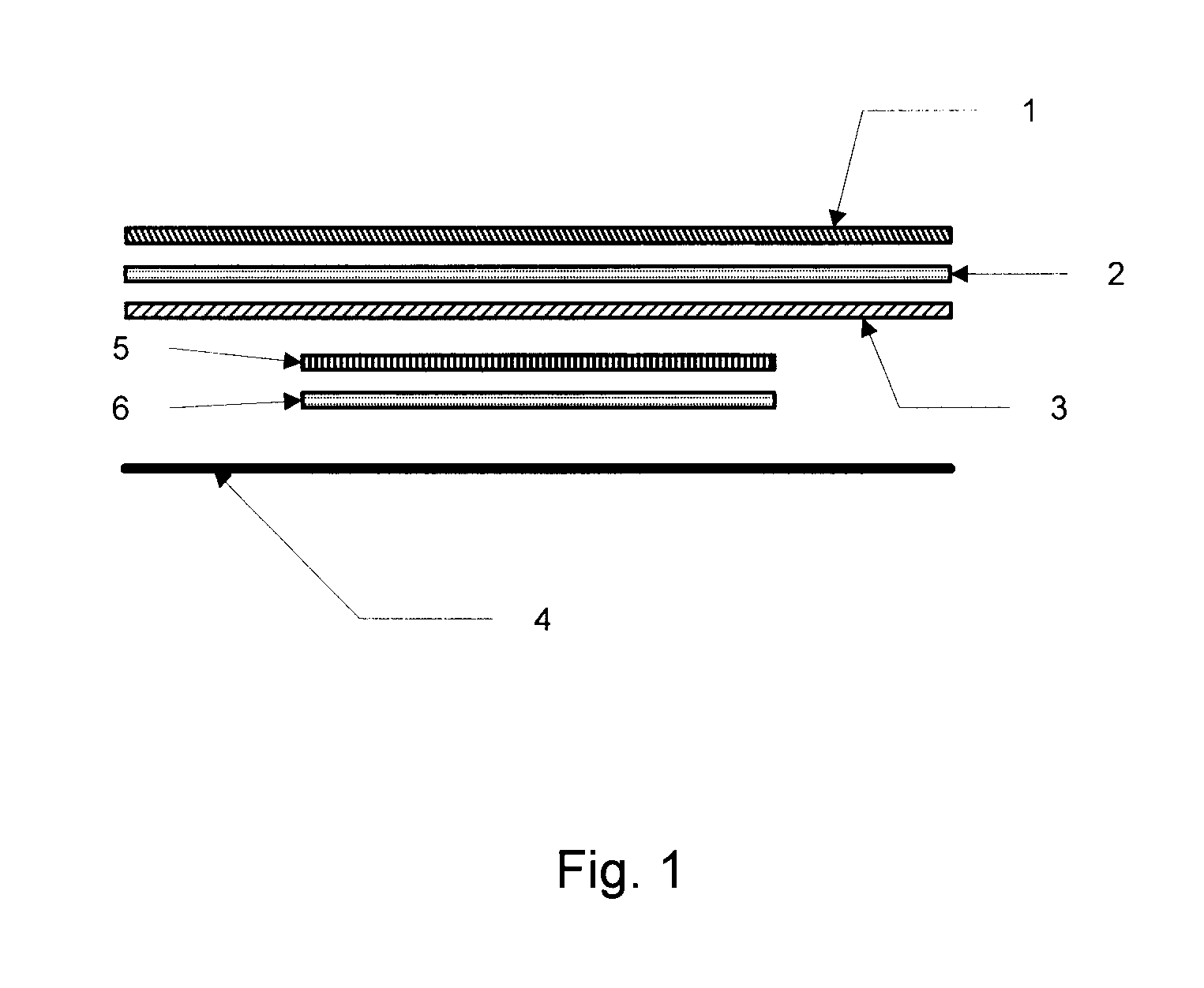
[0103]Example 1 is a description of one of the ways to fabricate a dermal delivery system of the invention. It will be appreciated that other ways can also be used. In this example, Part A illustrates preparation of Internal Backing / AI layer / Release Liner Laminate. Part B illustrates fabrication of a foam / acrylic PSA / PIB PSA overlay structure. Part C illustrates fabrication of an integrated device, or system, of the invention utilizing the laminates prepared in Parts A and B.
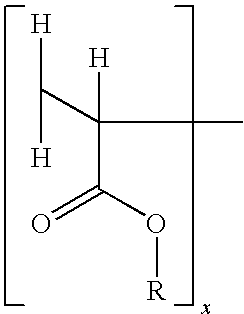
[0104]Part A. Fabrication of an Internal Backing / AI layer / Release Liner Laminate: After deaeration, an adhesive polymer composition comprising the AI and the volatile component(s) is applied to the backing layer material, and subsequently dried for a set time at a set temperature. In an alternative embodiment, the adhesive polymer matrix may be applied to a release liner instead of to the backing layer. Accordingly, reference herein to application of the adhesive po...
example 2
Control of Loss of Volatile Components
[0112]A transdermal hormone delivery device was prepared comprising an internal backing layer, an AI layer, and a release liner. The AI layer comprised, as a polymer matrix PSA: Duro Tak 87-4098; as a humectant: PVP / VA-5360; as skin permeation enhancers: dimethylsulfoxide (DMSO), lauryl lactate (Ceraphyl® 31), ethyl lactate, and capric acid; as the AI: levonorgestrel and ethinyl estradiol.
[0113]The device also comprised an overlay comprising a polyurethane spun bonded non-woven backing, which is able to absorb large amounts of the volatile excipients, DMSO and ethyl lactate, an acrylic PSA coated onto the polyurethane foam, and a PIB PSA coated on top of the acrylic PSA. The internal backing layer was composed of a Mylar polyester backing film onto which was coated an acrylic PSA containing the AI as well as the volatile excipients, DMSO and ethyl lactate at 8 wt % and 1.7 wt % nominal concentrations, respectively, and a non-volatile excipient, ...
example 3
[0117]Devices of a type described in Example 2 were analyzed for adhesion and irritation in a clinical study. Placebo patches or patches containing actives were applied to the skin of women for pre-determined time periods, among which were (1) one week, (2) two monthly treatment cycles, and (3) three monthly treatment cycles.
Adhesion Analyses:
[0118]A) Placebo patches applied for one week to 59 women: one out of 59 patches (1.7%) exhibited meaningful degree of detachment;
B) Active patches applied for two treatment cycles to 38 women: 12 out of 149 patches (8.1%) exhibited meaningful degree of detachment;
C) Placebo and active patches applied for three treatment cycles to 59 women: 13 out of 208 patches (6.3%) exhibited meaningful degree of detachment;
[0119]The mean cumulative total adhesion score (calculated per FDA Guidance) was 0.9 with an upper limit of 1.38 (placebo patch, one week, 59 women).
Irritation Analyses:
[0120]A) Placebo patches applied for one week to 54 wom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| perimeter | aaaaa | aaaaa |
| perimeter | aaaaa | aaaaa |
| perimeter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com