Trans-Differentiation And Re-Differentiation Of Somatic Cells And Production Of Cells For Cell Therapies
a somatic cell and transdifferentiation technology, applied in the field of somatic cell transdifferentiation and redifferentiation, can solve the problems of unanticipated plasticity in the ability to transdifferentiate stem cells, the inability to complete the conversion to a fully functional and stable and the inability to fully function and stabilize the different type of cell has never been demonstrated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070]Bovine fetal fibroblasts (BFFs) were grown to confluence and seeded onto 100 mm plates at approximately 250,000 cells / plate. Cells were grown in DMEM (Gibco) supplemented with 0.03% L-Glutamine (Sigma), 100 μM non-essential amino acids (Gibco), 10 units / L Penicillin-Streptomycin (Gibco), 154 μM 2-Mercaptoethanol (Gibco) and 15% FBS (HyClone). Four treatments were used:[0071]1. A control grown in the medium described above,[0072]2. DMEM with 2.5 μg / ml CB,[0073]3. DMEM with 5.0 μg / ml CB, and[0074]4. DMEM with 7.5 μg / ml CB.
[0075]Control cells were grown in the presence of DMSO alone to evaluate its effect on priming and trans-differentiation.
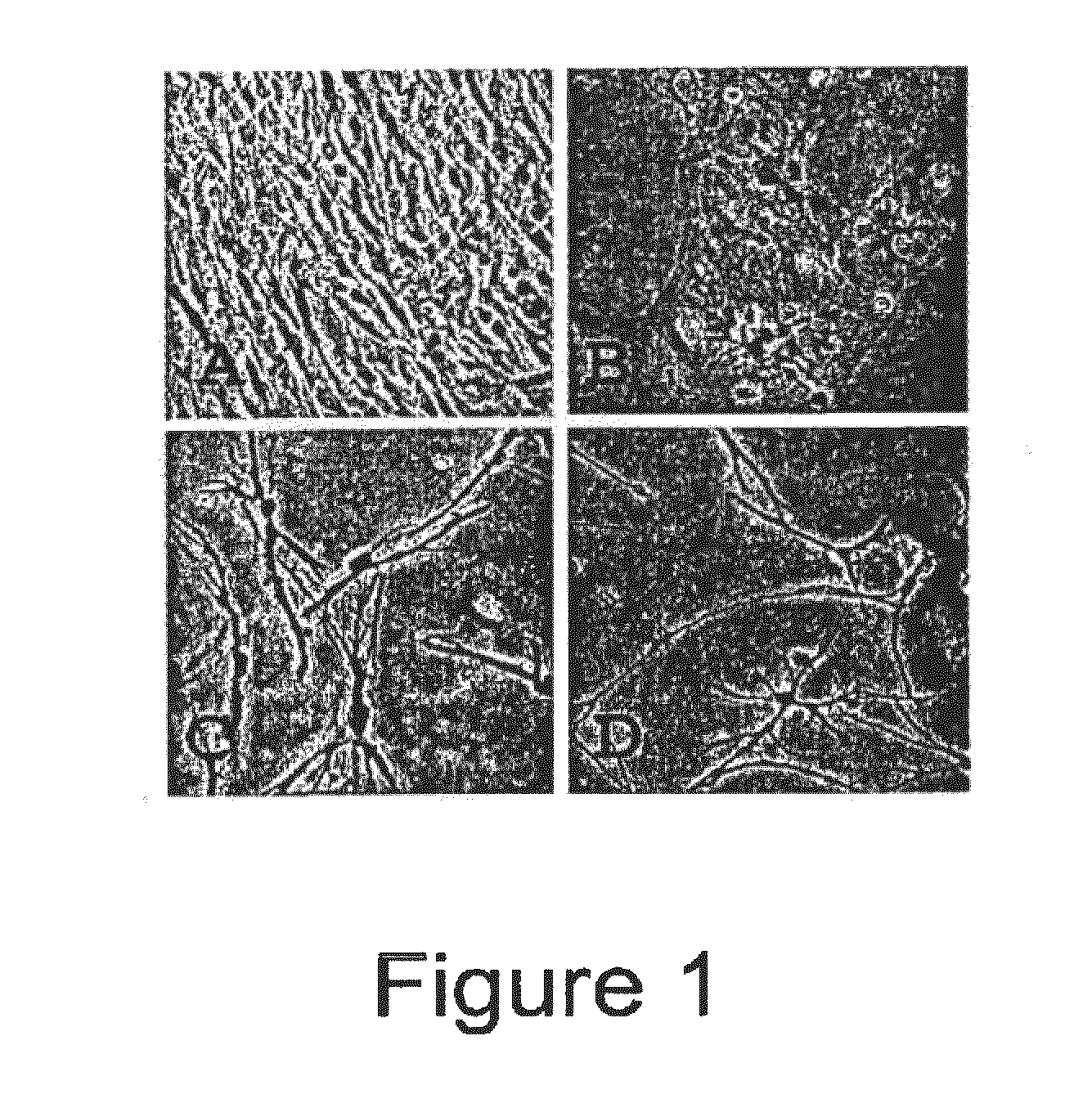
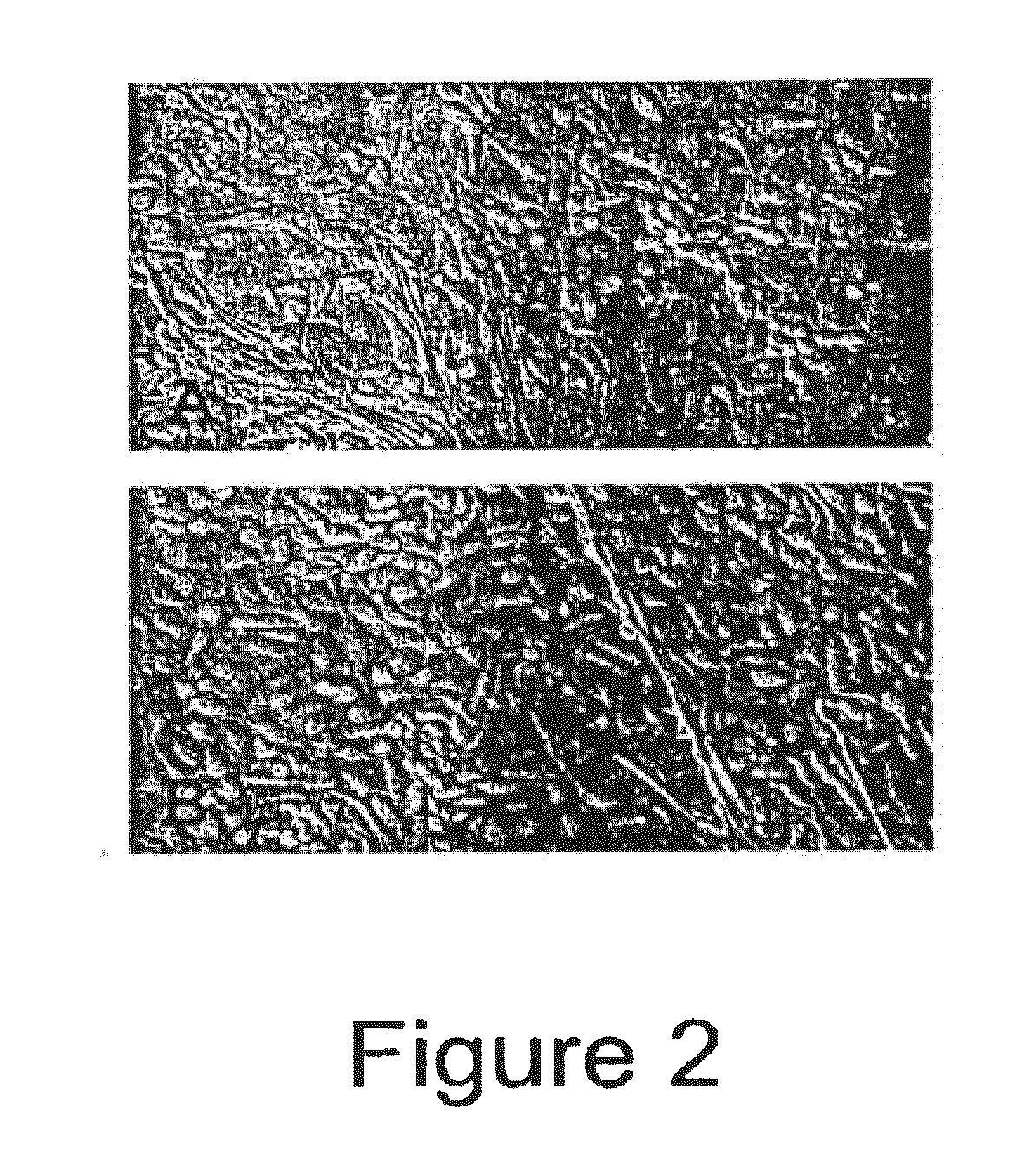
[0076]BFFs cultured in treatment 1 began to rapidly divide and grow to confluence as was expected. BFFs cultured in treatment 2 did not undergo cytokinesis, however did undergo nuclear division leading to multinuclear fibroblasts. BFFs cultured in treatments 3 and 4 began to change morphology and by day 2 of treatment began to take on the app...
example 2
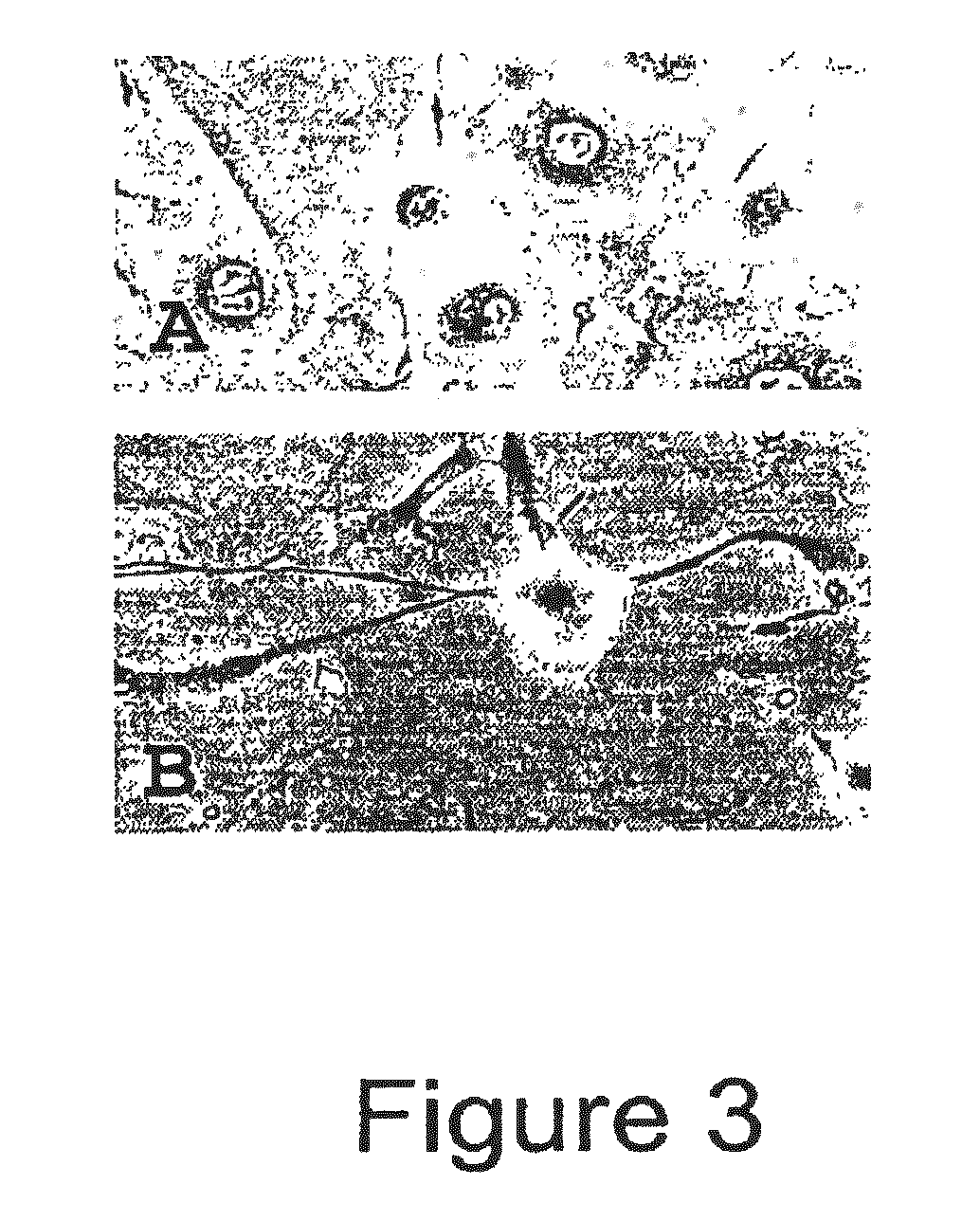
[0078]Bovine adult fibroblasts (BAFs) were treated in the manner described for BFFs in Example 1, with priming carried out using 10.0 μg / ml CB for 72 hours. Like BFFs, treatment of the BAFs with the priming agent and culturing them under conditions that induce neural differentiation caused the cells to undergo morphological changes toward a neuronal-like lineage. See FIGS. 3 and 4. Note that BFFs and BAFs acquire different morphologies of a neural type.
example 3
[0079]Transdifferentiation of human neo-natal fibroblasts. Fibroblasts were purchased from Cambrex company (Clonetics cell line # CC-2509) and were expanded in Iscove's Modified Dulbecco's Medium (IMDM, Gibco) supplemented with 20% fetal bovine serum (HyClone) at 37° C. in 5% CO2 and 5% O2. At passage 14, cells were weaned from serum by replacing medium every other day with half the concentration of serum over a 2-week period. When cells had been in the absence of serum for 48 hours, they were seeded at 50% confluency in 24-well dishes. 24 hours after passage, IMDM was removed and replaced with conducive medium (keratinocyte growth medium (KGM, Clonetics) was added to half of the cultures and neuro-progenitor growth medium (NPMM, Clonetics) was added to the other half). After 24 hours in conducive medium, 5 ug / ml cytochalasin B (CB, CalBiochem) was supplemented into half of each media group. Cells were cultured for an additional 72 hours at which point half of all groups were fixed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| plasticity | aaaaa | aaaaa |
| morphology | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com