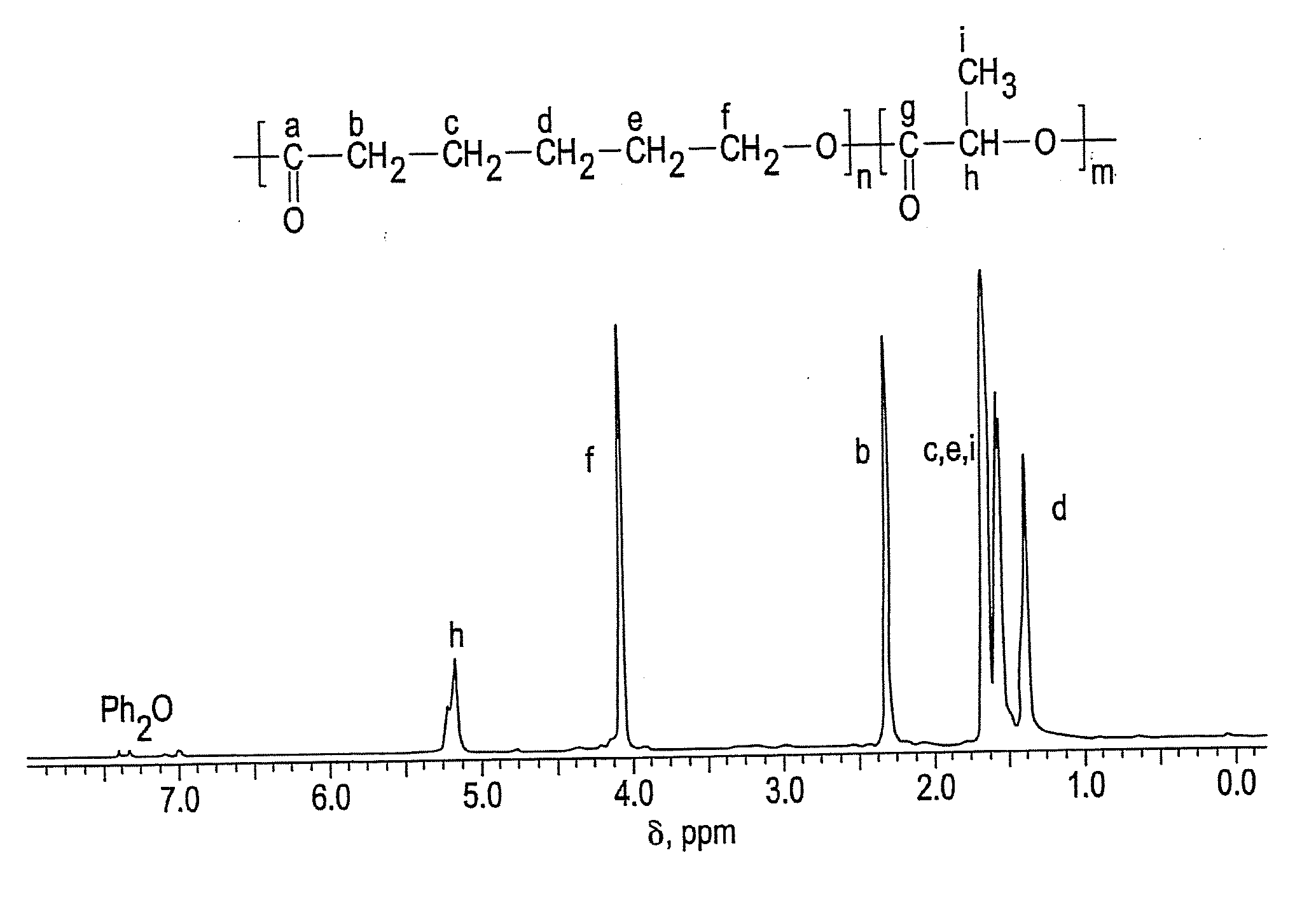
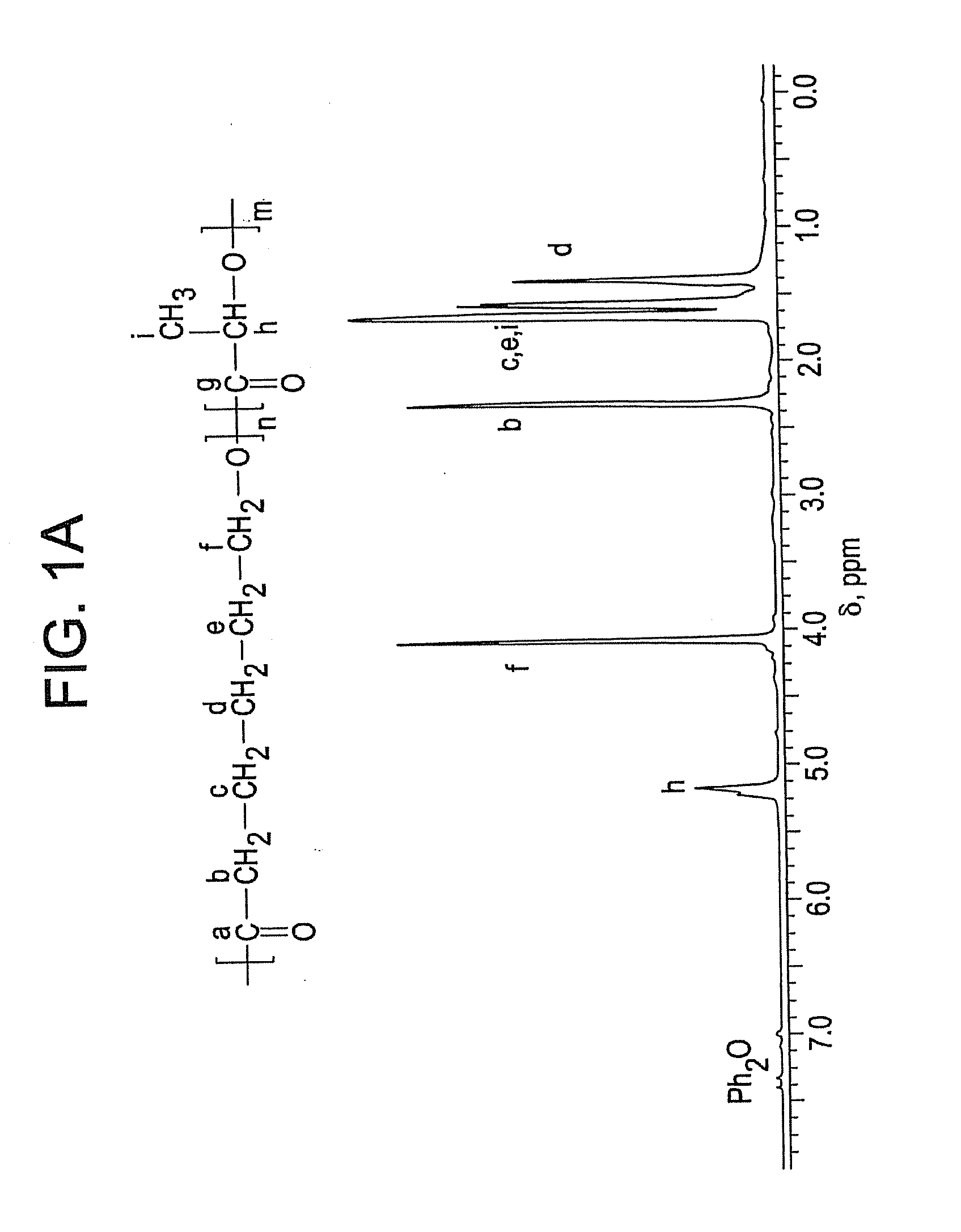
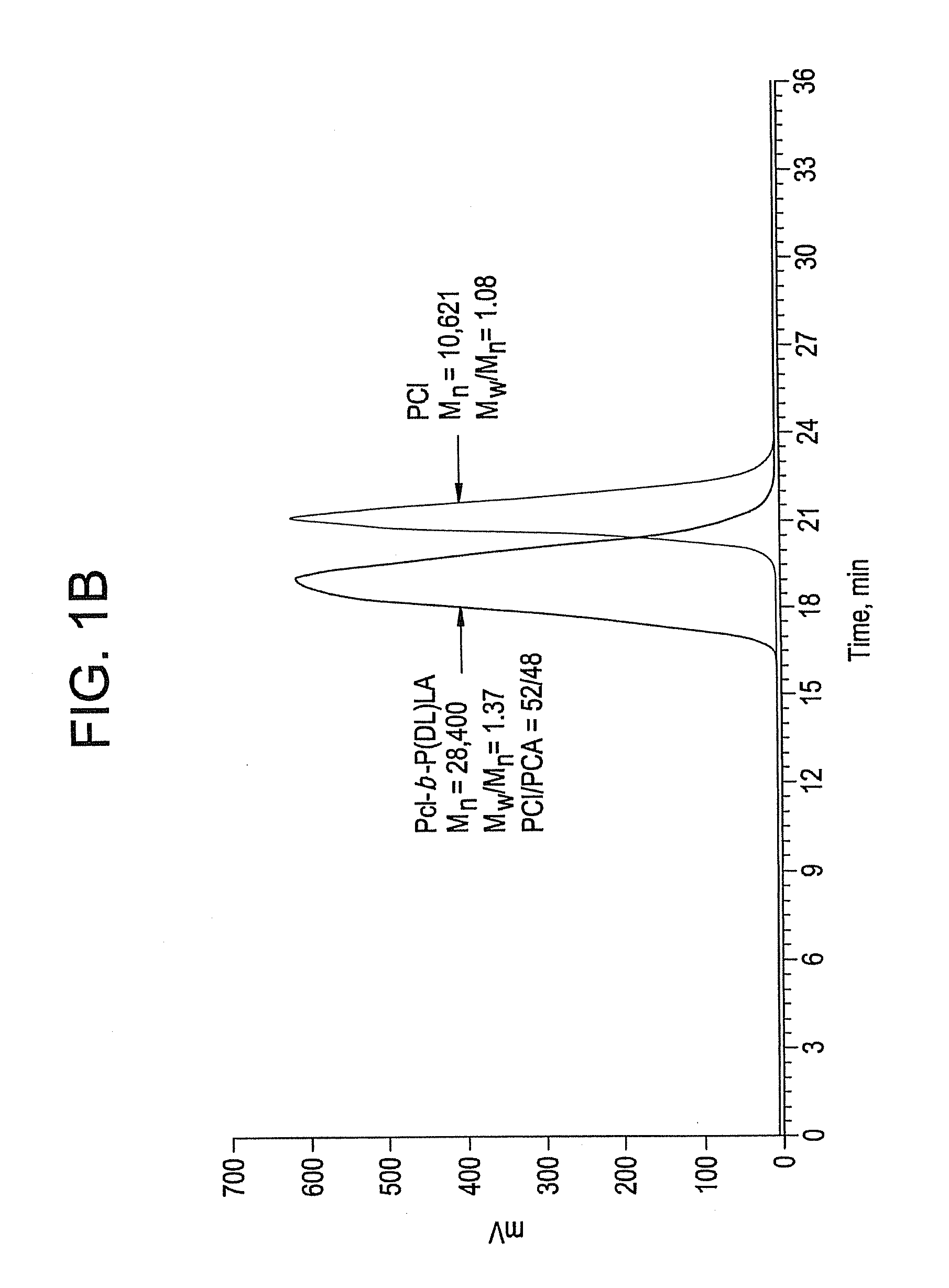
Ring-opening polymerization of cyclic esters, polyesters formed thereby, and articles comprising the polyesters
a technology of cyclic esters and polyesters, applied in the preparation of carboxylic acid esters, chemistry apparatus and processes, organic chemistry, etc., can solve the problems of limiting the use of polymers and polyyl alcohol, and polyyl alcohol has relatively low molecular weigh
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0063]This example describes the ring-opening polymerization of ε-caprolactone using Schwartz's reagent. Catalyst (Cp2ZrHCl, 23.27 milligrams (mg), 0.09 millimoles (mmol)), CL (1 milliliter (mL), 9.02 mmol) and toluene (1 mL) were added to a to a 25-milliliter Schlenk tube. The tube was degassed, filled with argon (Ar), and heated to 90° C. and maintained at the temperature for 2.5 hours. A colorless homogeneous solution was formed after less than one minute at 90° C. Periodically, samples were taken under Ar using an airtight syringe, divided into two parts. The first part was used for determination of percent conversion by 1H NMR, which indicated 93 percent conversion of monomer. The second part was used for molecular weight determination by GPC, which indicated a number average molecular weight of 36,577 and a polydispersity index of 1.2.
example 2
[0064]This example describes the synthesis of a high molecular weight polycaprolactone using Schwartz's reagent. 0.14 mL of Cp2ZrHCl stock solution in toluene (Cp2ZrHCl, 15 mg, 0.058 mmol in 3 mL toluene), CL (6 mL, 54.1 mmol), and toluene (18 mL) were added to a to a 250-mL Schlenk tube. The tube was degassed, filled with Ar, and refluxed at 150° C. under Ar. A colorless homogeneous solution was formed in less than one minute. Periodically, samples were taken under Ar using an airtight syringe and divided into two parts for percent conversion and molecular weight determination. These analyses indicated a 93% conversion of monomer, a number average molecular weight of 327,617 atomic mass units, and a polydispersity index of 1.13.
example 3
[0065]This example describes synthesis of a high molecular weight polycaprolactone using bis(cyclopentadienyl)zirconium dihydride. Cp2ZrH2 (10.0 mg, 0.045 mmol), CL (50 microliters (μL), 0.451 mmol) and toluene (4 mL) were combined in a Schlenk tube inside a glove box and heated to 90° C. A homogeneous dispersion (a cloudy mixture in which the cloudiness did not settle) was formed after 15 minutes. 162 μL, of the solution was added into a 250 mL Schlenk tube containing a pre-degassed mixture of CL (4 mL, 36.1 mmol) and toluene (16 mL). The tube was re-degassed, filled with Ar and was heated to 130° C. under Ar. A colorless homogeneous solution was formed. Periodically, samples were taken under Ar using an airtight syringe and divided into two parts for percent conversion and molecular weight determination. These analyses indicated a 66 percent conversion of monomer after 26 hours, a number average molecular weight of 278,778 atomic mass units, and a polydispersity index of 1.42.
PUM
| Property | Measurement | Unit |
|---|---|---|
| atomic mass | aaaaa | aaaaa |
| loss modulus | aaaaa | aaaaa |
| complex modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com