Immortalization of cells including neuronal cells
a neuronal cell and immortalization technology, applied in the field of immortalization of cells including neuronal cells, can solve the problems of unsatisfactory treatment of neuropathic pain, limited clinical application prospects, and limited clinical application prospects, and achieve the effect of increasing intracellular camp levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
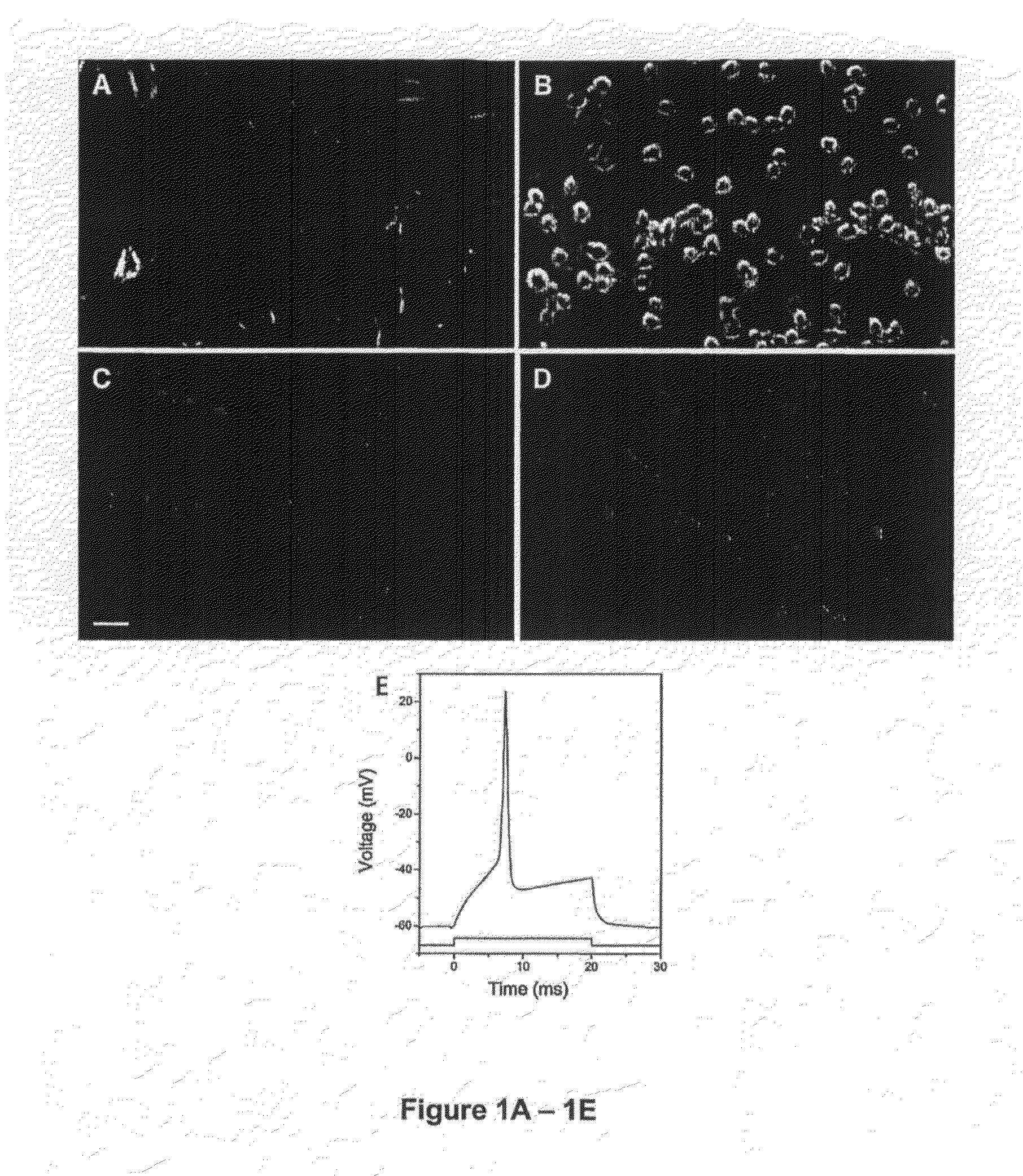
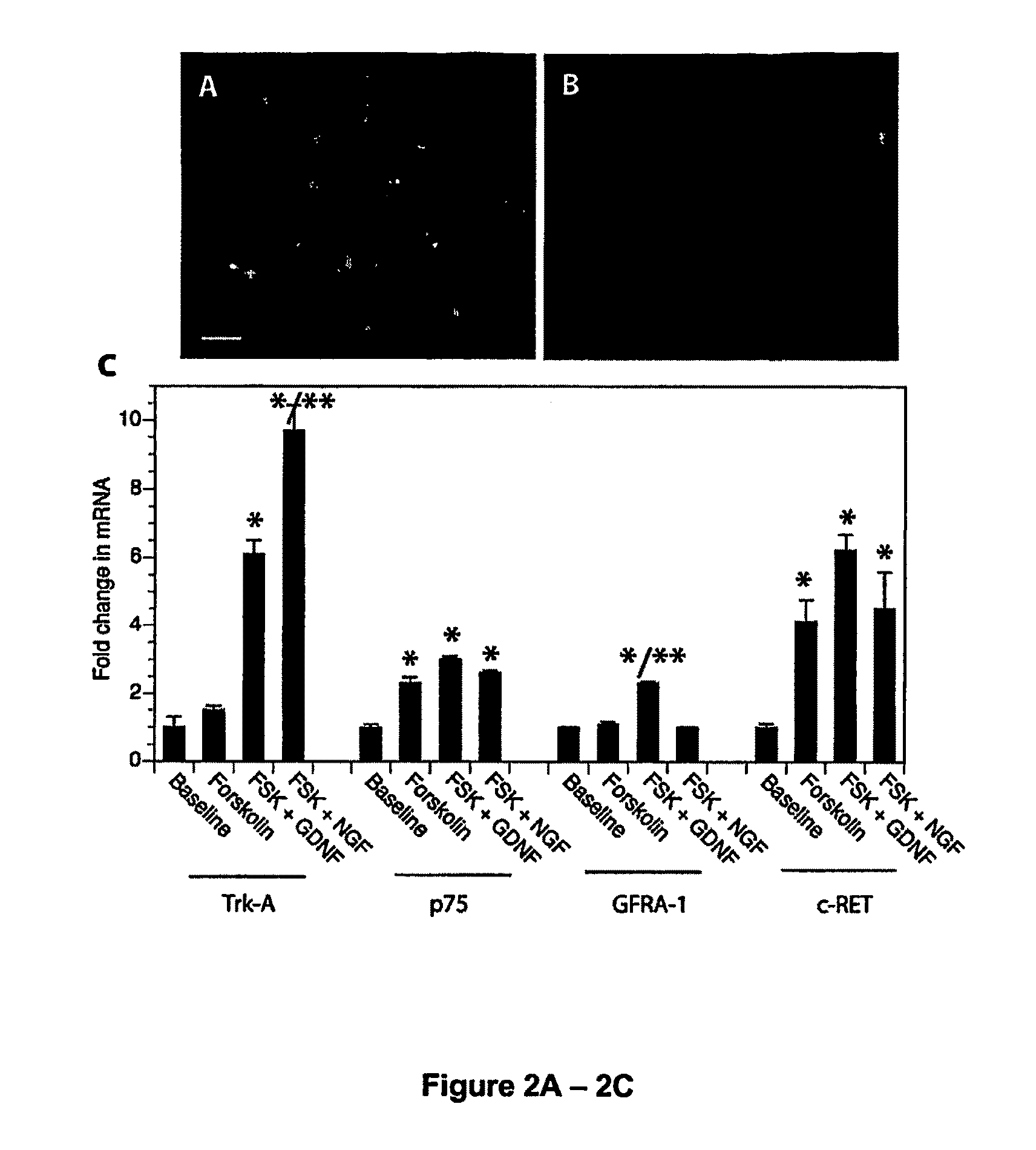
Generation and Characterization of DRG Neuronal Cell Line
[0067]Materials and Methods
[0068]Unless noted otherwise all reagents and materials were purchased from Invitrogen (Carlsbad, Calif.). Animal studies were conducted according the protocols approved by the institutional Animal Care and Use Committee.
[0069]Construction of SV40 Large T-Antigen and hTERT Expression Vectors
[0070]For cloning of the SV40 large T-antigen, plasmid construct pZipSV776-1 was used as the template for PCR amplification of the gene fragment coding for SV40 large T-antigen. PCR reaction was primed by oligonucleotides 5′-CACCGCTTTGCAAAGATGGATAAAG (sense) and 5′-AATTGCATTCATTTTATGTTTCA (anti-sense). Amplification was performed using the Expend High Fidelity PCR System (Roche, Indianapolis, Ind.). The PCR product was cloned into the pENTR / D-TOPO vector by directional TA-cloning. After confirmation of the sequence, the target SV40 large T-antigen gene was transferred into pLenti6 / V5-Dest vector using Gateway tech...
example 2
Generation and Characterization of Human Fetal Astrocytes and Schwann Cells
[0098]Human astrocytes or Schwann cells were prepared from aborted fetal tissues using standard cell culture techniques. Cells were cultured in Neurobasal media (Neurobasal MEM plus 10% FBS, 0.5 uM glutamin, 2% glucose, 1×B27 supplement) for 3-7 days in an incubator with 5% CO2 at 37° C. Cells were detached with a cell scraper and washed 2 times in Opti-MEM (Invitrogen, CA) and 0.8-1.0×106 cells were suspended in 100 ul Opti-MEM and dissociated with pipetting using a 200 μl tip.
[0099]Five μg pLenti6 / V5-DEST plasmid carrying hTERT gene (pLenti6 / hTERT) in 1.7 μl TE (pH8.0) and 15 μg pLenti6 / V5-DEST plasmid carrying SV40 large T antigen gene (pLenti6 / SV40) in 5 μl TE (pH8.0) were mixed with cells, transferred into a 0.2 cm gene-pulser cuvette (Bio-Rad, CA) and incubated for 5 minutes at room temperature. Gene Pulser Xcell Electroporation System (Bio-Rad, CA) was set at 850 μF×90V and the cells were pulsed once (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| action potentials | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com