Assays for Cancer Patient Monitoring Based on Levels of Analyte Components of the Plasminogen Activator System in Body Fluid Samples
a technology of plasminogen activator and cancer patient, which is applied in the direction of material analysis, drug composition, instruments, etc., to achieve the effects of reducing tumor size, avoiding patient subjection, and increasing the efficacy of treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Enzyme Linked Immunoassay (ELISA) to Measure Levels of uPA in a Body Fluid Sample
[0077]Serum and plasma samples were centrifuged in a tabletop microcentrifuge to remove all flocculent material and / or particulate matter. After centrifugation, the samples were analyzed without further treatment, or were stored at −70° C. for future analysis. The initial concentration of the serum sample examined did not exceed a concentration of 10% (i.e., a 1:10 dilution of sample in sample diluent), (Bayer Diagnostics / Oncogene Science uPA Microtiter ELISA assay kit manual).
[0078]The Bayer Diagnostics / Oncogene Science uPA Microtiter ELISA detects 25 pg / ml of uPA analyte in a test sample. The signal of the 25 pg / ml standard is greater than two times the zero (background) signal. In addition, the uPA ELISA is specific for the detection of uPA; for example, no cross-reactivity is detected against tPA and cathepsin D, even at high challenge doses.
Detailed Protocol
[0079]The uPA Microtiter ELISA kit (comme...
example 2
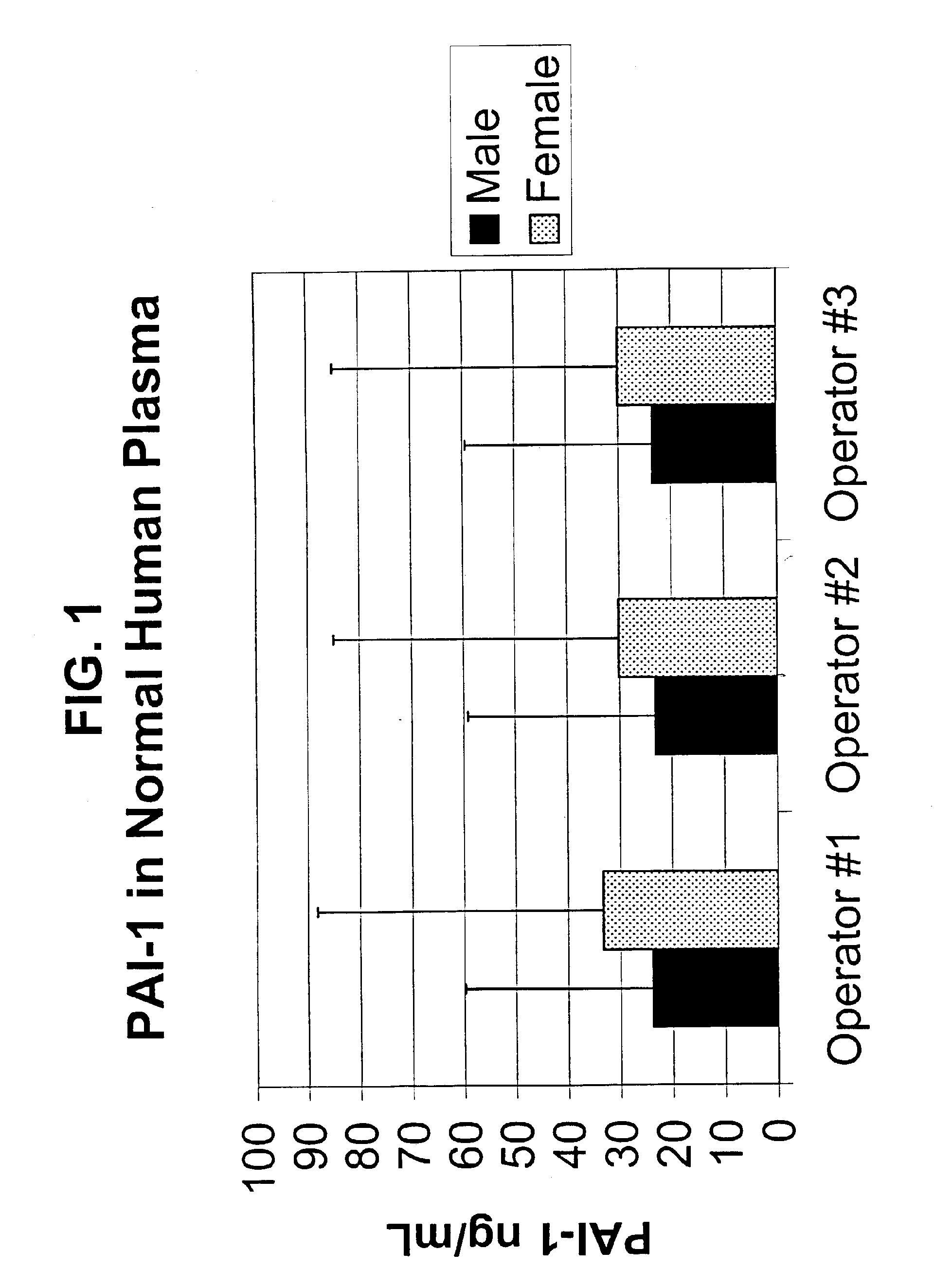
Enzyme Linked Immunoassay (ELISA) to Measure Levels of PAI-1 in Plasma
[0089]Plasma samples were centrifuged in a tabletop microcentrifuge to remove all flocculent material and / or particulate matter. Plasma samples were typically diluted 1:50 in sample diluent as described below and in accordance with the instructions accompanying the Bayer Diagnostics / Oncogene Science PAI-1 Microtiter ELISA assay. The contents of the PAI-1 Microtiter ELISA assay manual (Bayer Diagnostics / Oncogene Science, Cambridge, Mass.) are hereby incorporated by reference herein in their entirety.
[0090]The Bayer Diagnostics / Oncogene Science PAI-1 Microtiter ELISA detects 0.10 ng / ml of PAI-1 analyte in a test sample. The signal of the 0.10 ng / ml standard is greater than two times the zero (background) signal. In addition, the PAI-1 Microtiter ELISA is capable of quantifying PAI-1 in the active and latent forms, as well as in complex with uPA or tPA. The assay has been tested for cross-reactivity by challenging wi...
example 3
Enzyme Linked Immunoassay (ELISA) to Measure Levels of the uPA:PAI-1 Complex in Plasma
[0101]The Bayer Diagnostics / Oncogene Science Human uPA:PAI-1 Complex Microtiter ELISA detects 5 pg / ml of uPA:PAI-1 complex in a test sample. The signal of the 5 pg / ml standard is significantly higher than the zero (background) signal. In addition, the uPA:PAI-1 complex ELISA assay has been tested for specificity against PAI-2, tPA, ovalbumin, free uPA and free PAI-1 and showed no cross-reactivity against these proteins at high challenge doses.
Detailed Protocol
[0102]The uPA:PAI-1 complex Microtiter ELISA kit (commercially available from Bayer Diagnostics / Oncogene Science, Cambridge, Mass.) used in this Example has removable strips of wells so that the assay can be performed on multiple occasions, and only the number of wells needed are used. A standard curve was included each time samples were analyzed. The standard curve performed for each separate assay requires 12 wells (6 standards run in duplic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com