Multisite phosphorylated peptide (protein) recognizing compound and detection method, imaging method, alzheimer's disease diagnosing method and reagent kit using the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Luminescent Compound
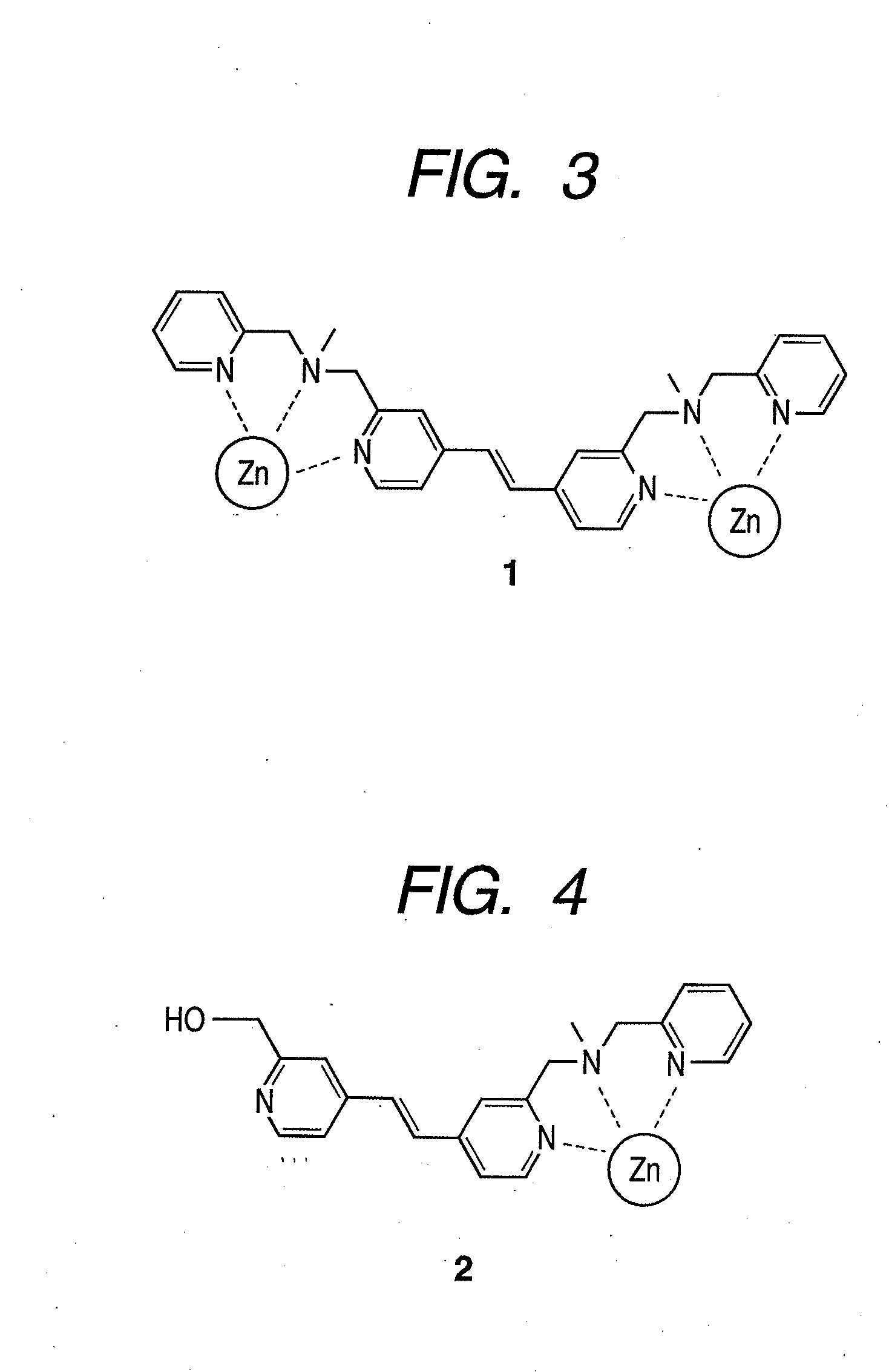
[0080]As the luminescent compound of the present invention, a Zn(Dpa)-stilbazole complex 1 (FIG. 3) represented by the above-mentioned formula (16) was synthesized by the following scheme. For comparison, a compound 2 having one Dpa (FIG. 4) was also synthesized. FIG. 5 illustrates a synthesis scheme.
Example 1(1)
Synthesis of Compound 3-1
[0081]5.0 g (31.7 mmol) of 2-pyridinecarboxylic acid hydrochloride and 15 mL of thionyl chloride were placed in a 100-mL three-neck recovery flask and stirred on an ice bath. 0.5 mL (31.7 mmol / l eq) of distilled water was slowly added dropwise to this mixture solution, and then reflux with heating was started. The reaction was followed by TLC (hexane / EtOAc=1 / 1), and completion of the reaction was confirmed three days later. The solvent was evaporated under reduced pressure, toluene was further added, and the mixture was evaporated under reduced pressure. When 30 mL of toluene was added to the residue, the mixture was ...
example 1 (
Example 1(17)
Conclusion
[0100]In summary, to make the skeleton of a compound molecule itself rigid, a Zn / Dpa complex was allowed to bind directly to a spacer (vinyl group), and a novel stilbazole compound in which the spacer was directly introduced at the fourth position of a pyridine ring in the Dpa was synthesized (compounds 1 and 2). Compound 1 recognizes and is crosslinked to phosphate groups existing distantly at the positions of (i, i+2) on the multisite phosphorylation sequence of a tau protein and binds in ratio of 1:1, and phosphorylation was successfully identified with dual wavelength fluorescence changes (Ka=˜105 M−1). Furthermore, it was found that compound 1 did not bind to two phosphate groups existing at the positions of (i, i+4) or (i, i+6) among a plurality of phosphate groups that existed and selectively recognized only two phosphate groups at the positions of (i, i+2). In a multisite phosphorylated peptide-recognizing compound in which a spacer binds to a nitrogen...
example 2
Synthesis of Luminescent Compound (BODIPY-Zn(Dpa))
[0102]As another luminescent compound according to the present invention, BODIPY-Zn(Dpa) represented by the formula (12) described above (compound 9) was synthesized as follows. FIG. 14 illustrates a synthesis scheme.
Example 2(1)
Synthesis of Compound 11
[0103]1.0 g (7.24 mmol) of 3,5-dihydroxy-benzaldehyde, 2.5 g (18.1 mmol, 2.5 eq) of potassium carbonate, 628.7 mg (7.24 mmol, 1.0 eq) of lithium bromide and 35 mL of dry DMF were placed in a 100-mL three-neck recovery flask, and the mixture was stirred at 100° C. Then, a solution of triethylene glycol monochlorohydrin in dry DMF (2.31 mL (15.9 mmol, 2.2 eq) / 10 mL) was added dropwise to the reaction solution, and the mixture was stirred continuously at 100° C. The reaction was followed by TLC (CHCl3 / MeOH=10 / 1), and completion of the reaction was confirmed five days later. Insoluble matters were isolated by filtration, and the filtrate was evaporated under reduced pressure. Ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com