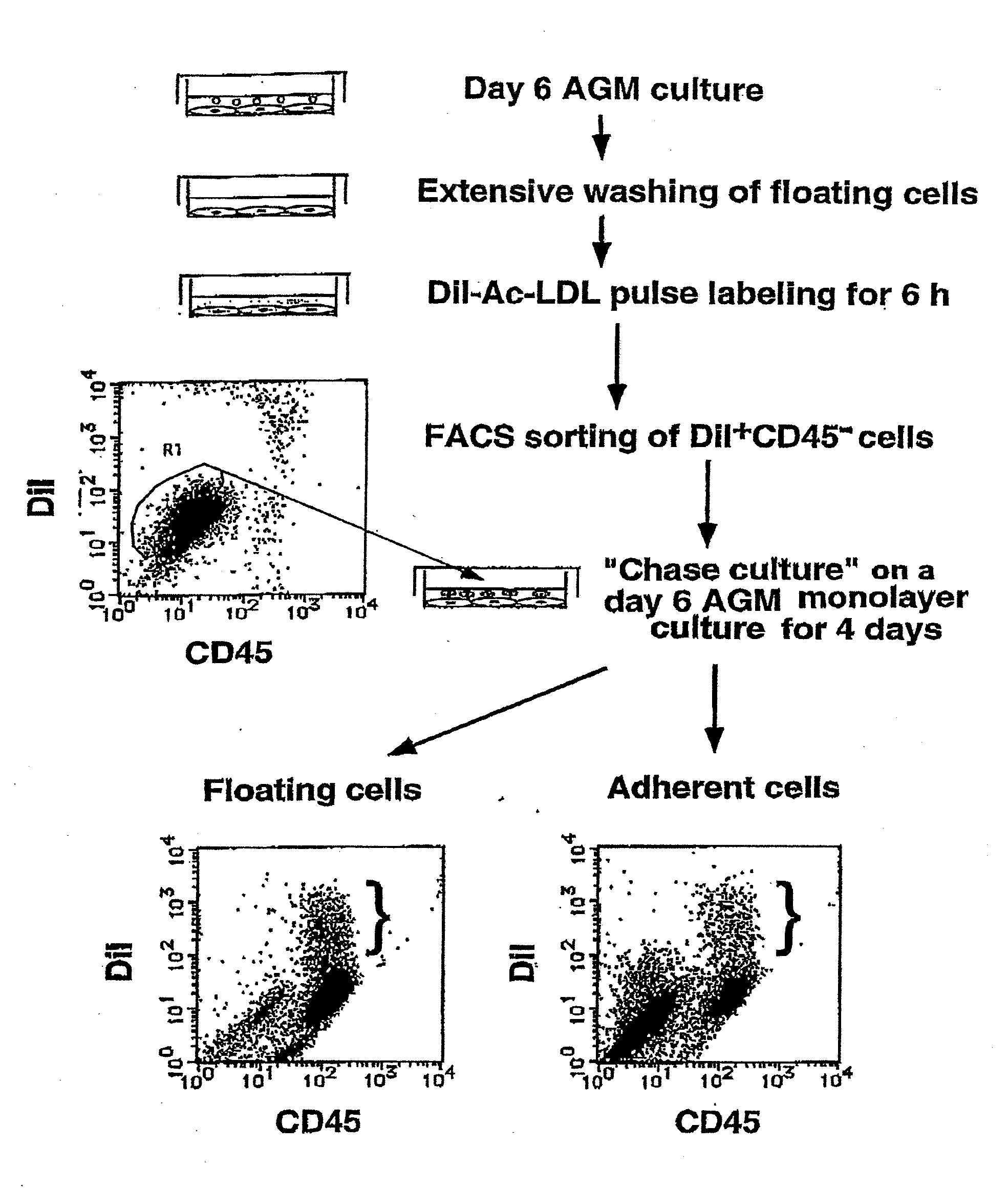
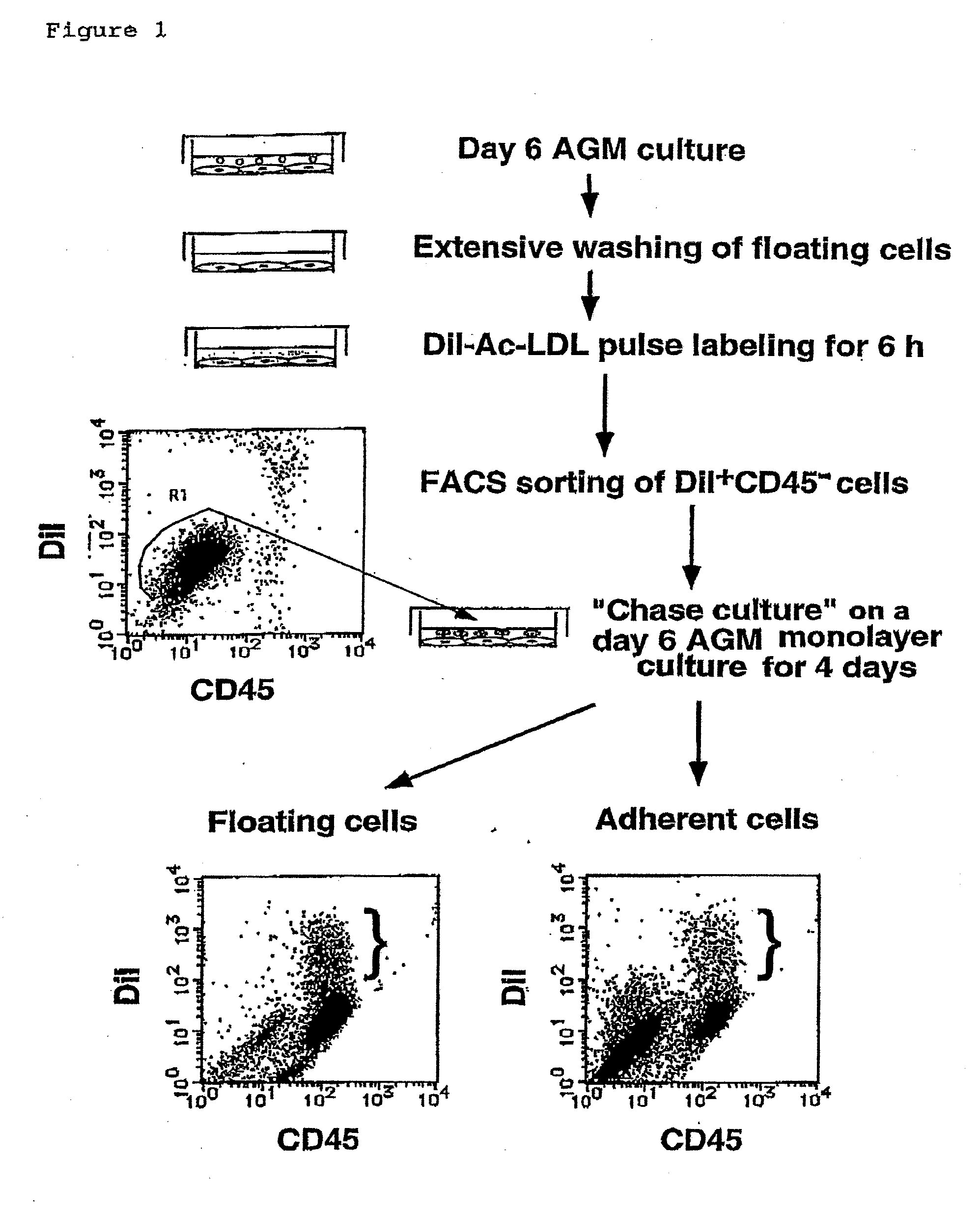
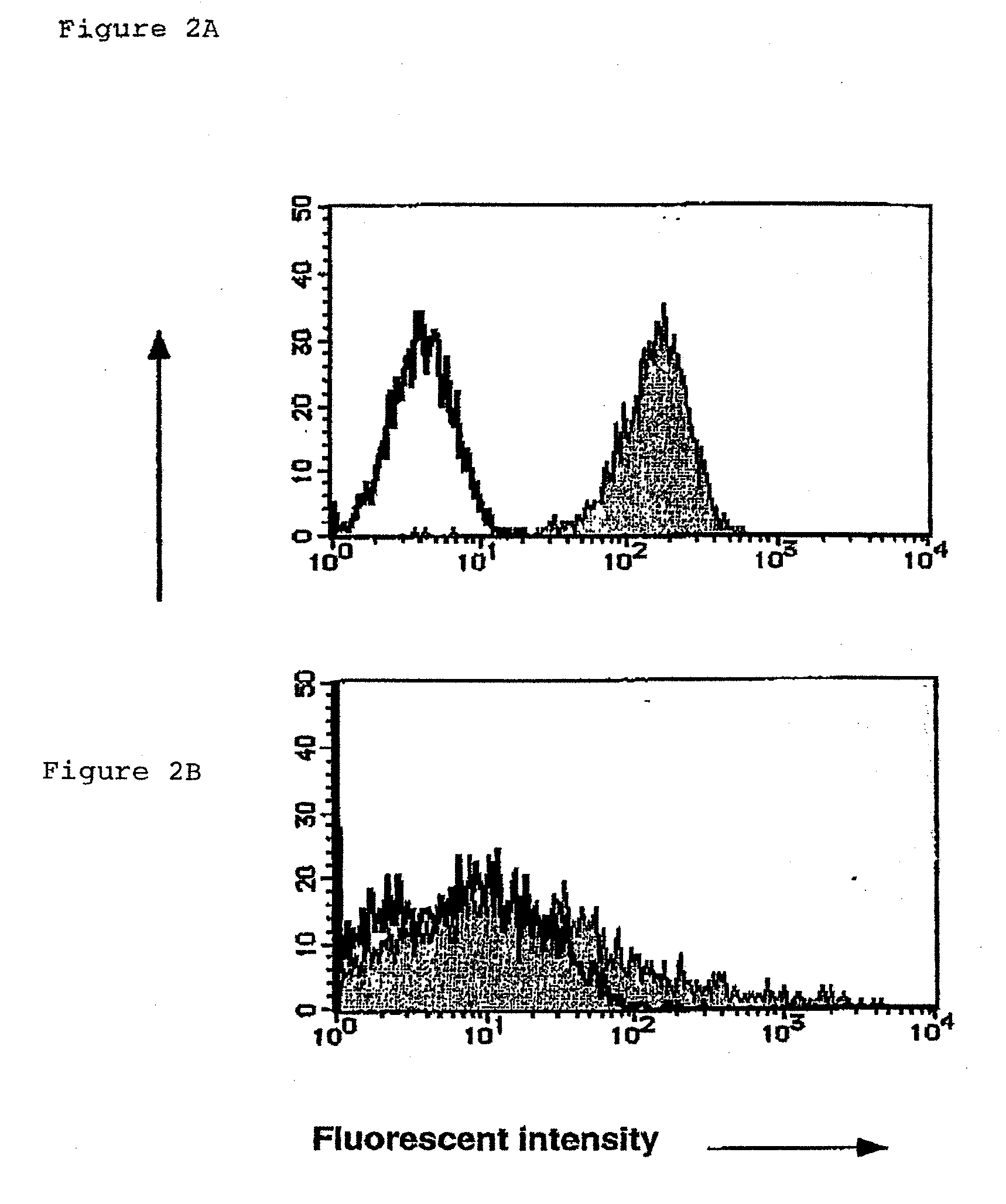
Method for Preparing Cell Fraction Containing Hemangioblasts
a cell fraction and hemangioblast technology, applied in the field of hemangioblasts, can solve the problems of unexplored nature of hemangioblasts, defects in both hematopoiesis and vasculogenesis, and still remains unknown how ltr-hscs in the yolk sac acquire full repopulation activity, so as to facilitate purification and detection of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Preparation of Monoclonal Antibodies Against Surface Antigens of Endothelial-Like Cells Derived from AGM Culture
[0119]To define hemangioblasts more precisely, the inventors aimed to obtain a specific antibody directed against hemangioblasts. By repeating the passage of adherent cells of the AGM culture in the presence of OSM, the inventors were able to establish a novel OSM-dependent endothelial-like cell line, LO. LO cells exhibit characteristics very similar to those of endothelial-like cells in the AGM culture, such as endothelial-like morphology, incorporation of DiI-Ac-LDL, and production of hematopoietic cells. The inventors used the LO cells as immunogens to raise monoclonal antibodies against cell surface antigens on LO cells as follows.
[0120]Wistar rats (Nihon SLC) were immunized with 107 of LO cells in the presence of Freund's complete adjuvant (WAKO, Osaka, Japan) according to the standard immunization procedure (Hockfield, S. et al. (1993) “Selected Methods for Antibody ...
example 3
Molecular Cloning of Mouse PCLP1 Molecule as a Possible Hemangioblast Antigen
[0122]Next, using a standard expression cloning strategy with COS7 cells and 10B9 monoclonal antibody, the inventors isolated a cDNA clone encoding the 10B9 antigen.
[0123]Expression cloning of a cDNA encoding the 10B9 antigen was carried out by using COS7 cells as previously described (Harada, N. et al. (1990) Proc. Natl. Acad. Sci. USA 87, 857-861) except that magnetic beads conjugated with anti-rat IgG antibody (Dynabeads M-450) (Dynal, Oslo, Norway) were employed instead of plate panning. Briefly, COS7 cells were fused with spheroplasts of the cDNA plasmid library of LO cells (Tanaka, M. et al. (1999) Blood 93, 804-815) and stained with 10B9 antibody followed by Dynabead selection. Plasmid DNA mixture was harvested from the beads, amplified in E. coli and re-transfected into COS7 cells. This procedure was repeated 4 to 5 times until a single band of cDNA insert was recovered. As a result, the inventors i...
example 4
Expression of PCLP1 on the Endothelial-Like Cells in the AGM Culture
[0128]The inventors examined the expression of PCLP1 on the endothelial-like cells in the AGM culture by immunostaining with 10B9 anti-PCLP1 antibody. Cultured AGM-derived cells in plastic plates were fixed with 1% paraformaldehyde (PFA)-PBS at room temperature for 15 minutes and incubated with anti-PCLP1 10B9 antibody at 10 μg / ml at 4° C. over night. After incubation with peroxidase-conjugated anti-rat IgG (Amersham), signals were visualized by 3,3′-diaminobenzidine (DAB) as previously described (Hara, T. et al. (1998) Dev. Biol. 201, 144-153).
[0129]As the inventors expected, PCLP1 was detectable on endothelial-like cells (FIG. 5A to C), but not on fibroblastic cells (data not shown) in the AGM culture. The endothelial-like cells, defined by their polygonal cell morphology and incorporation of DiI-Ac-LDL, were further fractionated by fluorescent activated cell sorting (FACS) using anti-PCLP1 and anti-CD45 antibodie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com