Methods and compositions relating to HDAC 4 and 5 regulation of cardiac gene expression
a gene expression and gene technology, applied in the field of molecular biology, can solve the problems of sudden death, heart failure, dilated cardiomyopathy,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0259]Preparation of primary rat cardiomyocytes. cardiomyocyte cultures are prepared by dissociation of 1-day old neonatal rat hearts and were differentially plated to remove fibroblasts. To induce the hypertrophic response, AngII and PE are added to cardiomyocyte cultures at 10 mM and 10 μM, respectively, in serum-free M199 media. The culture media containing either agonist is changed every 12 hours for a period of 72 hours.
[0260]Immunocytochemistry. To visualize sarcomeric organization in primary cardiomyocytes, anti-α-actinin mouse monoclonal antibody is used (Sigma). Cells are washed in 1×PBS, fixed in 3.7% paraformaldehyde for 5 minutes, washed three times with 1×PBS and then pre-blocked in 1×PBS containing 2% horse serum, 2% BSA, and 0.1% NP40 for 30 minutes. Anti-α-actinin antibody is added at a dilution of 1:800 in fresh pre-block solution and incubated for an additional 30 minutes. Subsequently, cells are washed three times in 1×PBS with 0.1% NP40. Anti...
example 2
[0263]The Role of MEF2 in Cardiac Gene Expression.
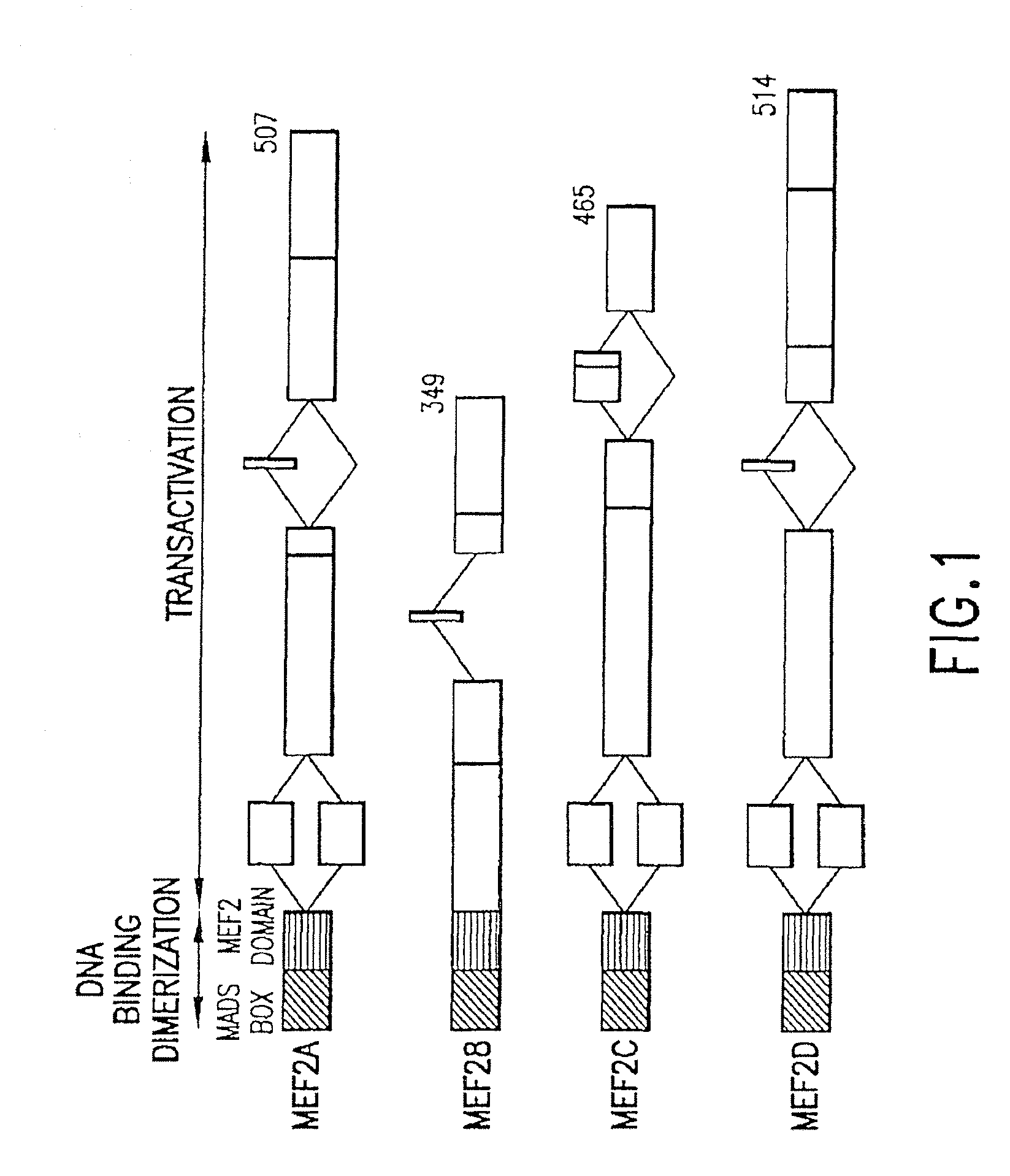
[0264]Structure-function studies. There are four vertebrate MEF2 genes, whose products are schematized in FIG. 1. Through extensive mutational analyses, the functional domains of the MEF2 proteins have been characterized (Molkentin et al., 1995; Martin et al., 1993; Molkentin et al., 1996a; 1996b). These studies demonstrate that the N-terminal MADS-box mediates DNA binding and dimerization. The adjacent MEF2 domain influences DNA binding affinity and interactions with myogenic bHLH proteins, and the C-terminal regions of the MEF2 factors contain multiple independent transcriptional activation domains.
[0265]Cooperative activation of muscle transcription by MEF2 and myogenic bHLH factors. In the skeletal muscle lineage, MEF2 acts combinatorially with members of the MyoD family of bHLH transcription factors to activate muscle gene transcription. It has been demonstrated that the MADS-box of the MEF2 proteins interacts directly with the ...
example 3
Induction of MEF7 Activity In Vitro by Hypertrophic Signaling
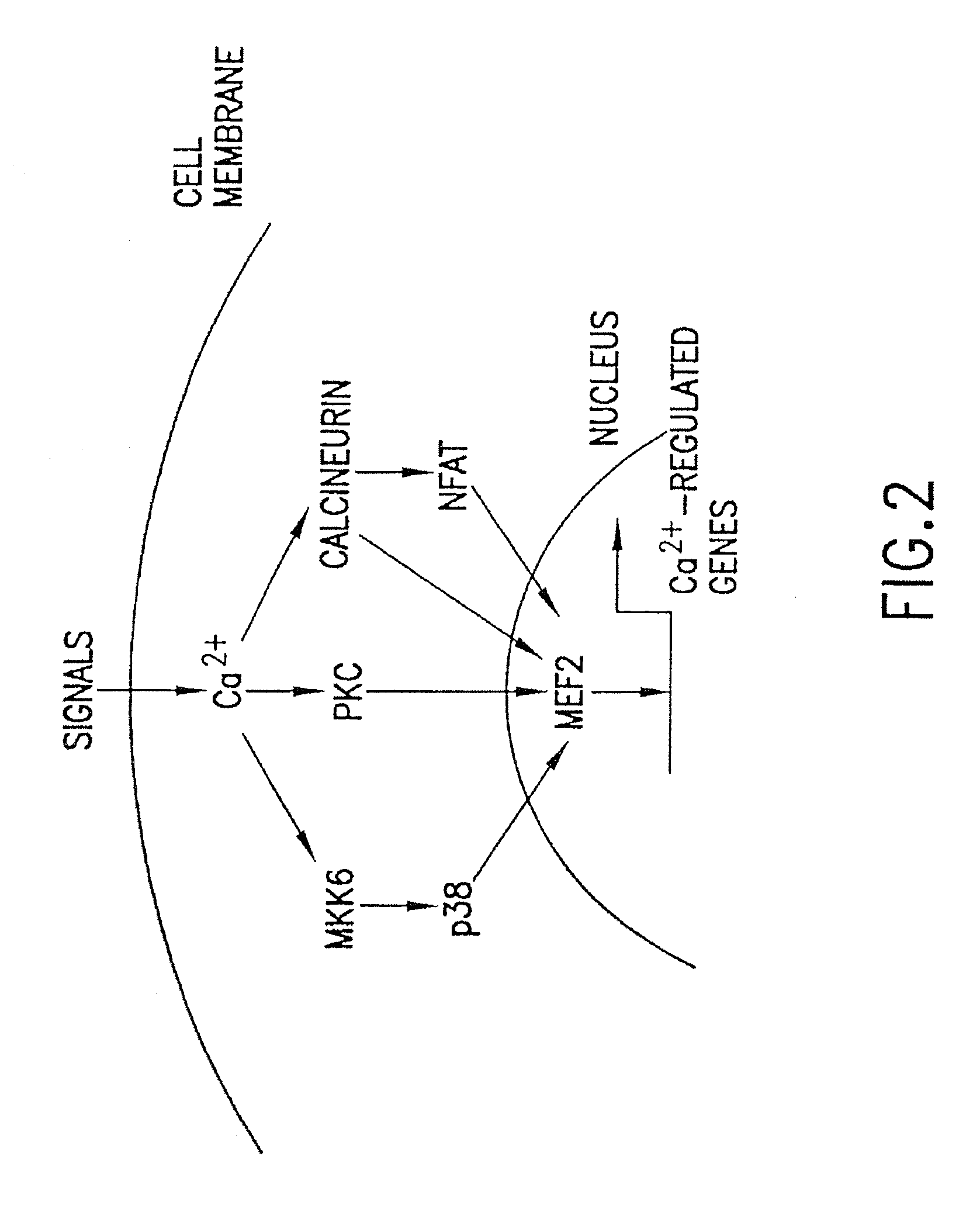
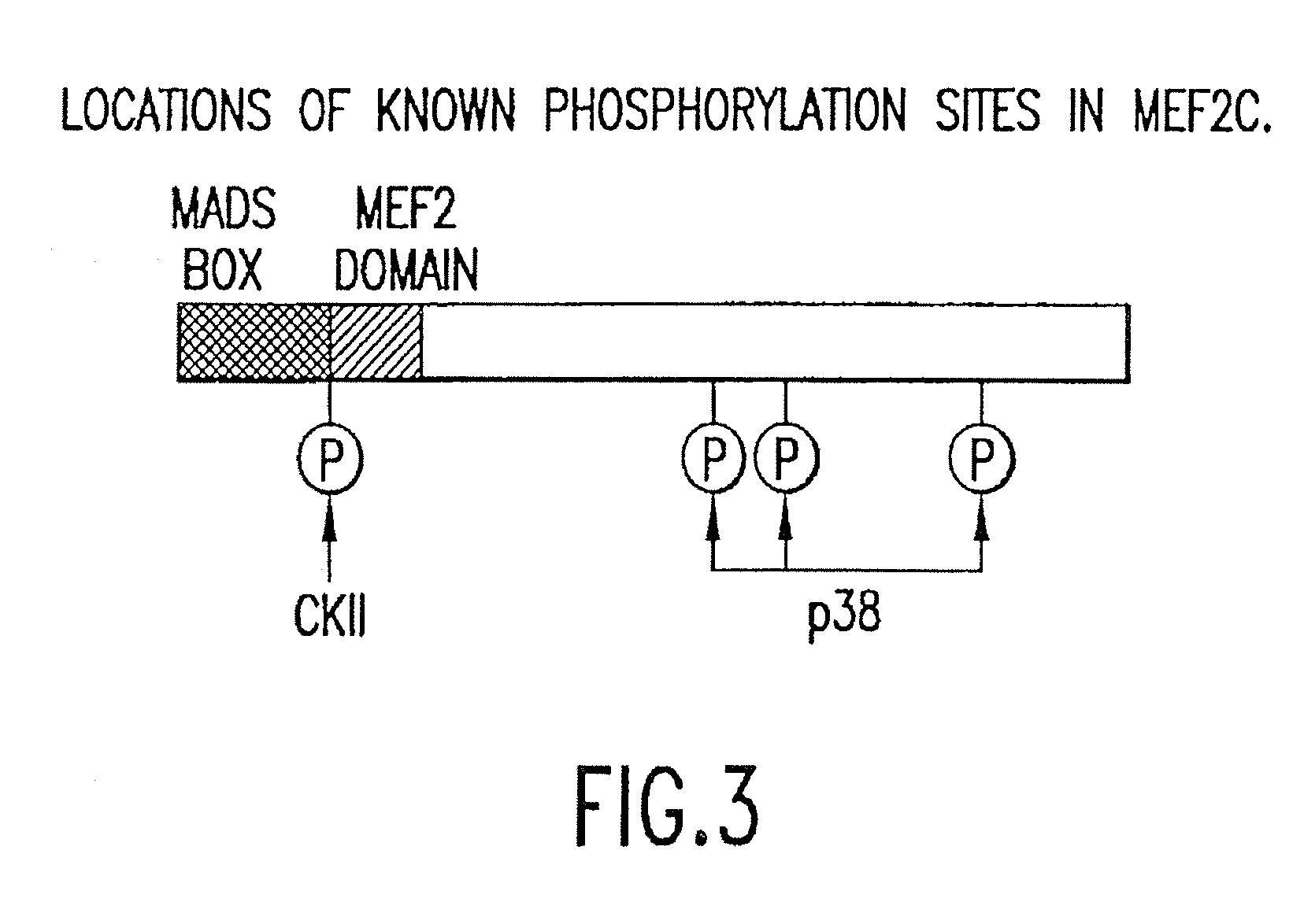
[0269]In light of the ability of MEF2 to respond to calcium-dependent signal transduction pathways in T cells, the inventors have investigated whether the same pathways also activate MEF2 in cardiomyocytes. As shown in FIG. 4, activated calcineurin or CaMKIV can upregulate a MEF2-dependent luciferase reporter gene in transfected cardiomyocytes and together these calcium-sensitive signaling enzymes synergistically activate MEF2-dependent gene expression. In DNA binding assays, an increase in MEF2 DNA binding activity in response to activated calcineurin and CaMKIV is not observed suggesting that the increase in MEF2 transcriptional activity reflects a post-translational mechanism. When the C-terminus of MEF2C, which contains the transcription activation domains (TADs), but lacks the MADS and MEF2 domains required for DNA binding and dimerization, is fused to the DNA binding domain of the yeast transcription factor GAL4, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| delay time | aaaaa | aaaaa |
| delay time | aaaaa | aaaaa |
| delay time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com