Fluorogenic ph sensitive dyes and their method of use
a technology of fluorogenic ph and dyes, which is applied in the direction of diaryl/triaryl methane dyes, organic compounds of group 3/13 elements, peptides, etc., can solve the problems of limiting the applicability of dyes described, affecting the applicability of dyes, and affecting the stability of solution,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 101
Synthesis of pH Sensor 104 Having the Methoxy pKa-Enhancing Group (Scheme 101)
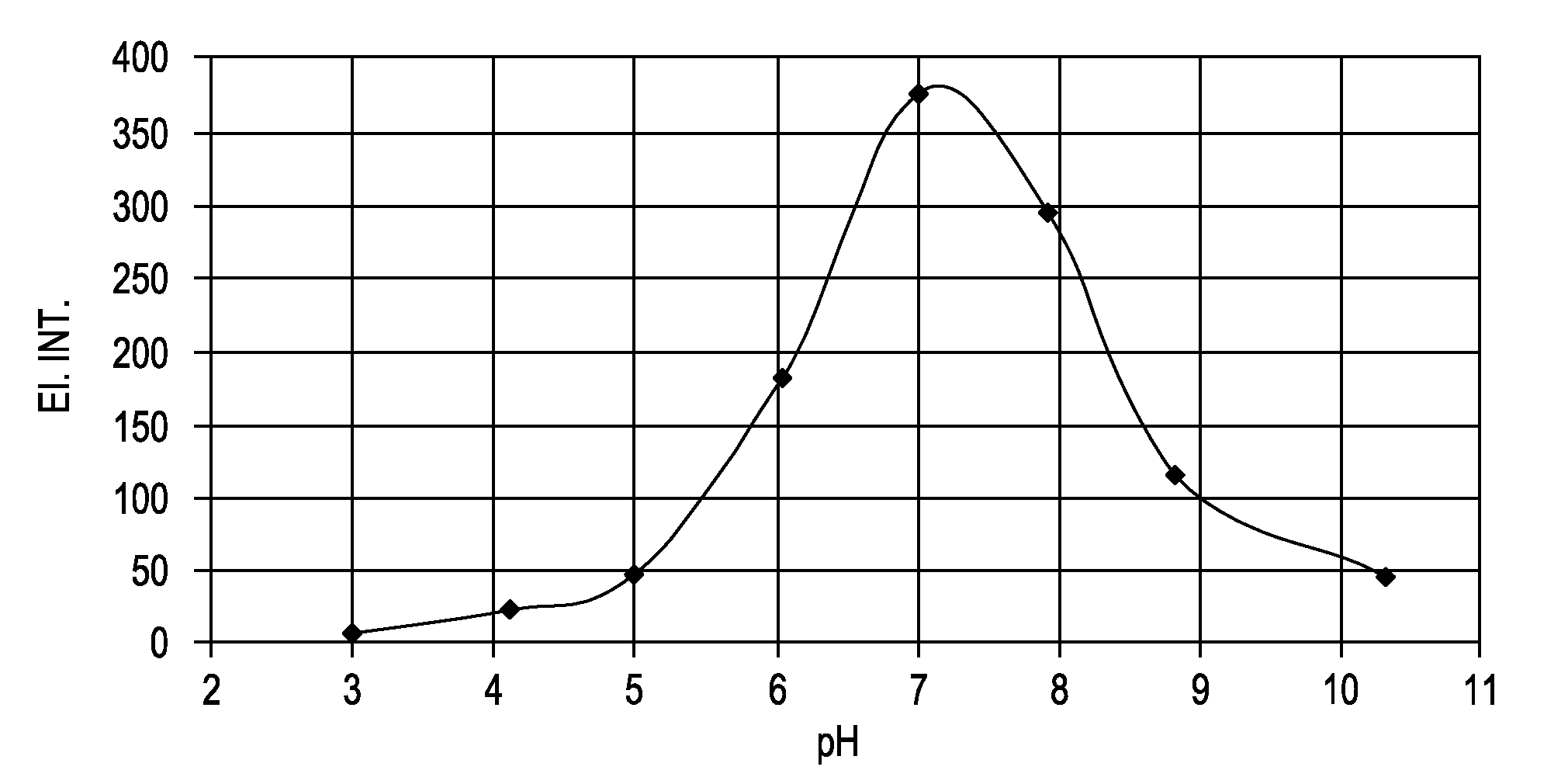
[0642]
[0643]Compound 104. To a stirred suspension of ketone 101 (94 mg, 0.333 mmol) in dry chloroform (10 mL), oxalyl chloride (30 μL, 0.33 mmol) was added upon cooling to 0-5° C. The resulted red solution was stirred for 1 h, then N,N-diethyl-m-anisidine (60 mg, 0.33 mmol) was added. The reaction was allowed to warm to rt, stirred for 16 h and diluted with CHCl3 (60 mL).
[0644]Chloroform solution was shaken with sat. NaHCO3 (40 mL) until water layer turned almost colorless. The organic layer was washed with sat. NaHCO3 (20 mL) and extracted with 10% HCl (2×30 mL). The combined acid extract was washed with CHCl3 (2×15 mL; discarded), the aqueous solution was saturated with sodium acetate and extracted with CHCl3 (4×30 mL). The extract was washed with brine (30 mL), and evaporated. The crude product was purified by chromatography on silica gel column (2×40 cm bed, packed with 10% MeOH and 1% AcOH in CHCl3) e...
example 102
Synthesis of pH Sensor 109 Having Two Methoxy pKa-Enhancing Groups and Compound III with a Labeling Succinimidyl Ester Moiety (Scheme 102)
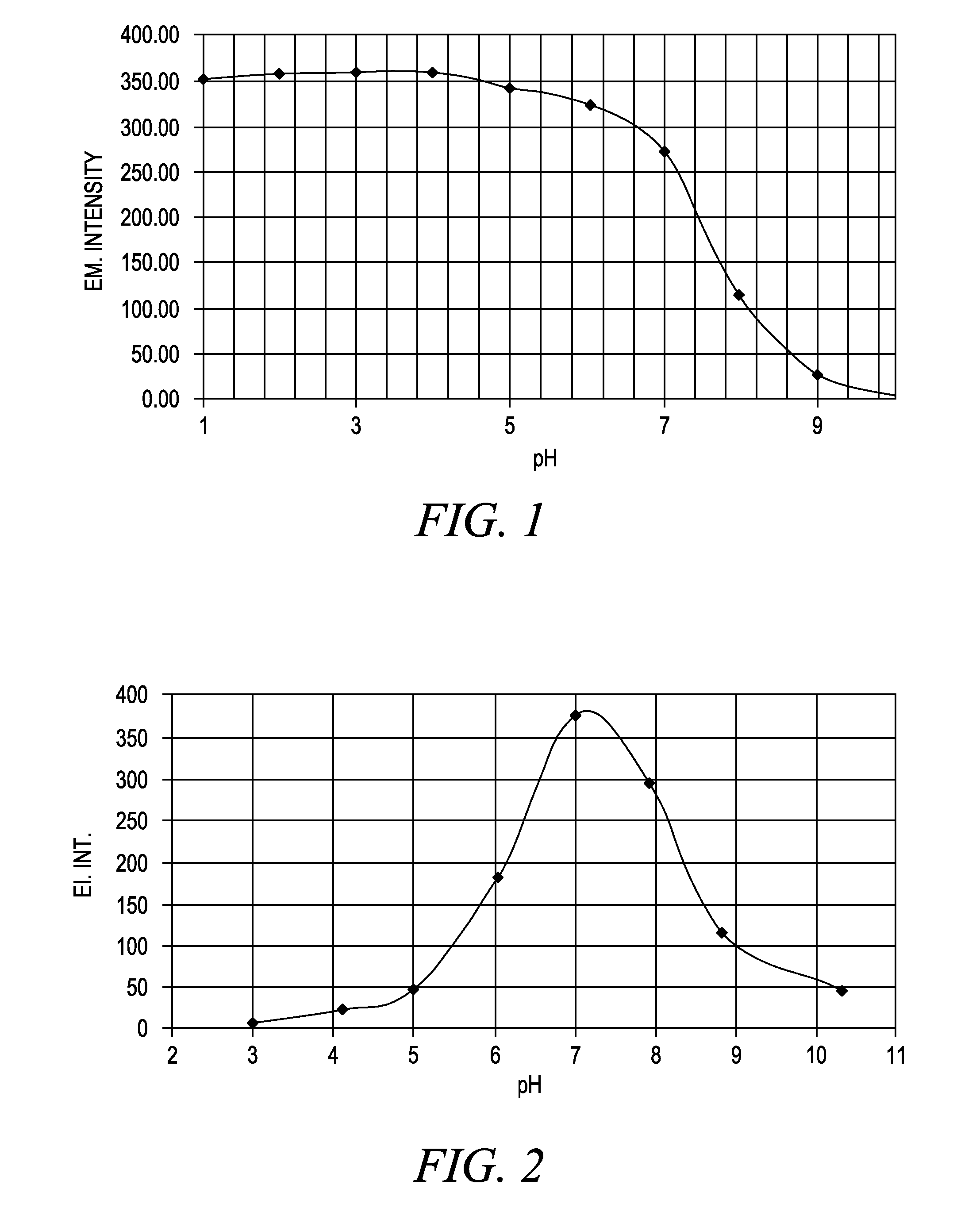
[0645]
[0646](2,5-Dimethoxyphenyl)acetamide (106). To a stirred solution of 2,5-dimethoxyaniline (105) (1.00 g, 6.52 mmol) and DIEA (1.71 mL, 9.80 mmol) in CHCl3 (20 mL) was added acetic anhydride (0.93 mL, 9.8 mmol). The reaction mixture was stirred for 1.5 h, washed with water (50 mL), 5% HCl (50 mL), brine (50 mL), dried over Na2SO4, and evaporated to give acetate 106 (1.33 g, 100%) as a dark oil.
[0647]2,5-Dimethoxy-N-ethylaniline (107). To the solution of acetamide 106 (1.27 g, 6.5 mmol) in dry THF (10 mL) a borane-THF complex (1M in THF, 58.5 mL, 58.5 mmol) was added upon ice-water cooling. The mixture was allowed to warm to rt and stirred under reflux for 16 h. The mixture was cooled to 0° C. and carefully decomposed with MeOH (40 mL), and then the mixture was heated to reflux and stirred for 1 h. Upon cooling, the solution was concentrated a...
example 103
Synthesis of pH Sensor 118 Having Methoxy pKa-Enhancing Group and Compound 120 with a Labeling Succinimidyl Ester Moiety (Scheme 103)
[0652]
[0653]Ethyl 4-(4-benzyloxy-2-nitrophenyloxy)butanoate (113). 4-(Benzyloxy)-2-nitrophenol (112) (2.00 g, 9.39 mmol), K2CO3 (1.30 g, 9.42 mmol), NaI (0.70 g, 4.7 mmol) and ethyl 4-bromobutyrate (2.70 mL, 18.7 mmol) in DMF (10 mL) were stirred for 3 hrs at 70° C. The reaction mixture was cooled to rt, diluted with EtOAc (100 mL), washed with water (4×40 mL), 5% HCl (3×40 mL), brine (30 mL), dried over Na2SO4 and evaporated to give ester 113 (2.10 g, 62%) as a brown oil.
[0654]4-(4-Hydroxy-2-nitrophenyloxy)butanoic acid (114). Ester 113 (1.50 g, 4.17 mmol) was stirred under reflux with 30 mL of water and 30 mL of conc HCl for 1.5 hrs. Upon cooling the mixture was diluted with water (150 mL) and extracted with EtOAc (3×40 mL). The extract was washed with brine (50 mL), dried over Na2SO4 and evaporated. The crude product was dissolved in 1M KOH (30 mL),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com