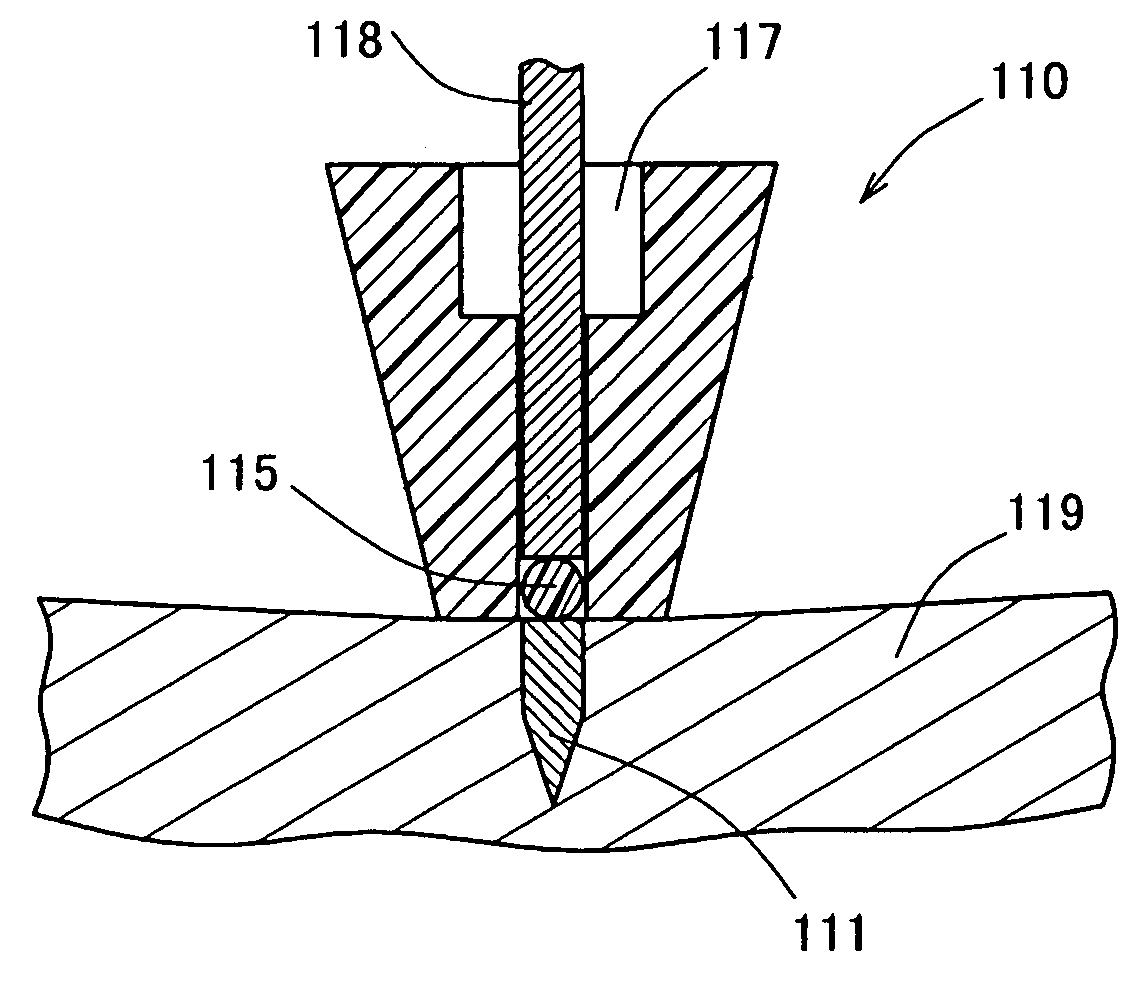
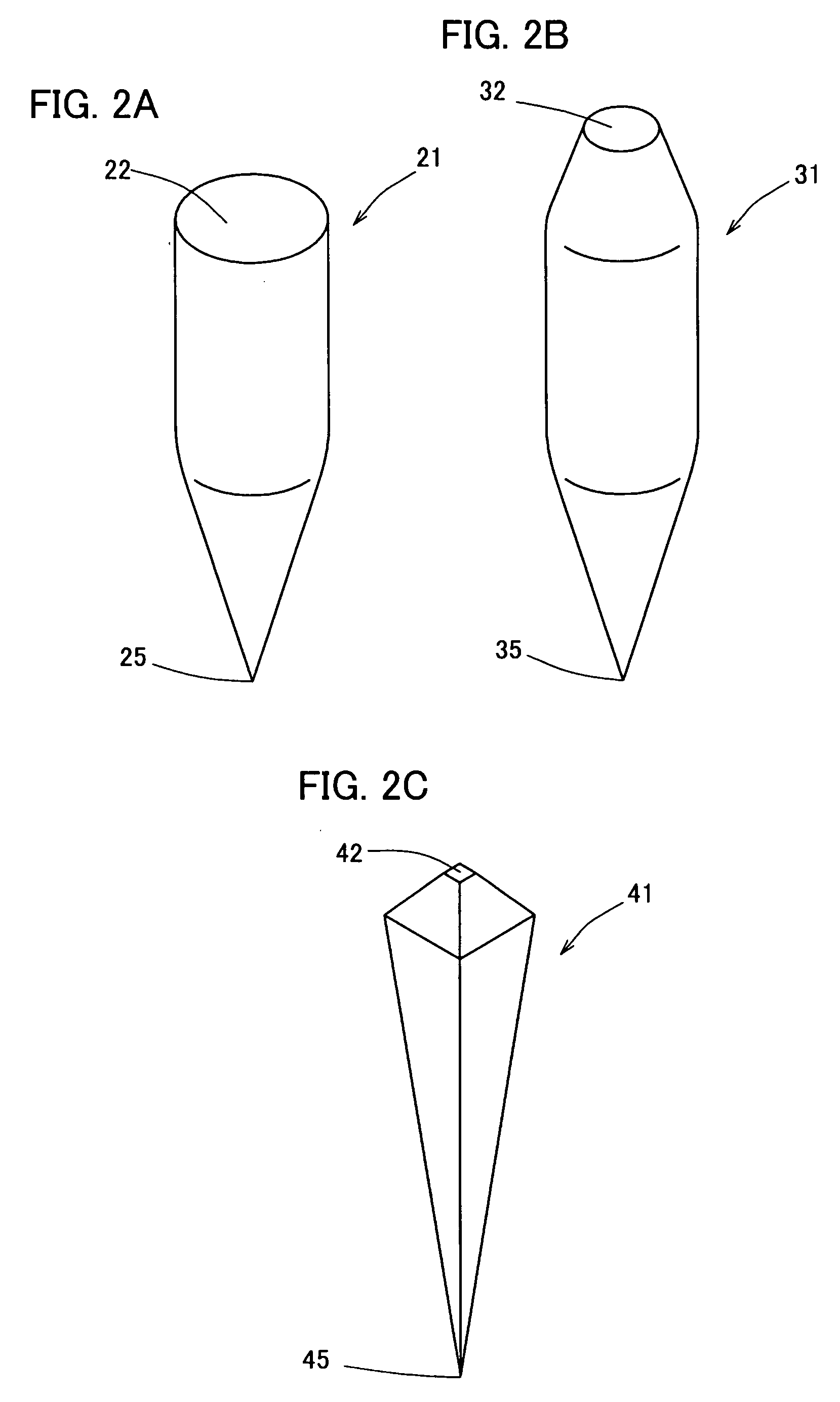
Percutaneously Absorbable Preparation, Percutaneously Absorbable Preparation Holding Sheet, and Percutaneously Absorbable Preparation Holding Equipment
a technology of absorbable preparations and percutaneous administration, applied in the direction of drug compositions, immunological disorders, metabolism disorders, etc., can solve the problems of difficult absorption of macromolecular drugs such as insulin limited use of these percutaneous preparations, and difficult systemic therapy with drugs through the percutaneous route, etc., to achieve convenient administration, efficient percutaneous administration, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0147]This example illustrates a percutaneously absorbable preparation having a needle-like and filamentous shape and containing interferon (active substance) held in a base consisting of human serum albumin.
[0148]About 0.2 mL of distilled water was added to 150 mg of human serum albumin (Sigma) to be dissolved. The solution was mixed well to give a base paste consisting of human serum albumin. To this base paste, μL of interferon alpha injection solution “Sumiferon” (Trademark, 6,000,000 units / mL, Sumitomo pharmaceuticals), corresponding to 60,000 IU, was added and well mixed so that interferon was held in the base paste. To the base paste holding interferon, a top of a glass stick of which diameter was about 3 mm was attached. Thereafter, the top was gradually pulled apart so that the base paste attaching to the top has a needle-like or filamentous shape. The needle-like or filamentous base paste was solidified by drying at low temperature to give a percutaneously absorbable prepa...
example 2
[0150]This example illustrates a percutaneously absorbable preparation having a needle-like and filamentous shape and containing interferon (active substance) held in a base consisting of bovine serum α-acid glycoprotein (AAG).
[0151]About 50 μL of distilled water was added to 50 mg of bovine AAG (Sigma) to be dissolved. The solution was mixed well and water was evaporated to give a base paste consisting of AAG. To this base paste, 10 μL of interferon alpha injection solution “Sumiferon” (Trademark, 6,000,000 units / mL, Sumitomo pharmaceuticals), corresponding to 60,000 IU, was added and well mixed so that interferon was held in the base paste. A Percutaneously absorbable preparation having a needle-like or filamentous shape was made with a glass stick in the same way as Example 1.
example 3
[0152]This example illustrates a percutaneously absorbable preparation having a needle-like and filamentous shape and containing FITC-labeled albumin (active substance) held in a base consisting of human serum albumin. FITC-labeled albumin was used as a model of vaccine.
[0153]FITC-labeled albumin was prepared by labeling human serum albumin with fluorescein isothiocyanate (FITC). On the other hand, about 0.2 mL of distilled water was added to 130 mg of human serum albumin (Sigma) to be dissolved. The solution was mixed well to give a base paste consisting of human serum albumin. To this base paste, 20 mg of FITC-labeled albumin was added and well mixed, so that FITC-labeled albumin was held in the base paste. To the base paste holding FITC-labeled albumin, a top of a polypropylene stick of which diameter was about 2 mm was attached. Thereafter, the top was gradually pulled apart so that the base paste attaching to the top has a needle-like or filamentous shape. The needle-like or fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com