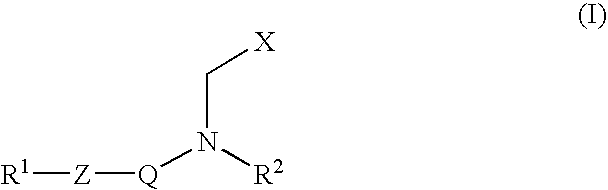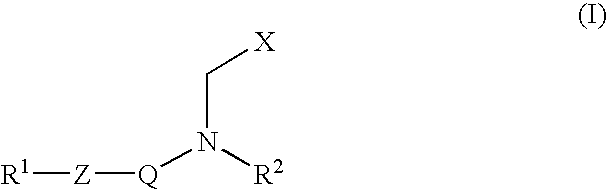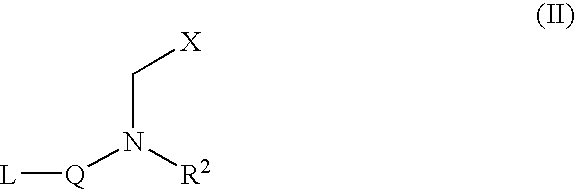Inhibitors of matrix metalloproteinase
a matrix metalloproteinase and inhibitor technology, applied in the direction of peptide/protein ingredients, drug compositions, immunological disorders, etc., can solve the problems of more and achieve the effects of stimulating inflammation, increasing inflammatory cell infiltration, and increasing enzyme production and subsequent tissue damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0074]
[(4′-Cyano-1,1′-biphenyl-4-yl)(methylsulfonyl)amino]acetic acid
[0075]A solution of [(4-bromophenyl)(methylsulfonyl)amino]acetic acid (intermediate 3, 20 mg, 65 μmol) in dimethoxyethane (1 mL) was added in one portion to a mixture of 4-cyanophenyl-boronic acid (9.5 mg, 65 μmol) and fibrecat FC1001 (2.71% Pd; 25 mg, 6.5 μmol) in a Smith microwave reaction vial. Aqueous sodium carbonate solution (1.0 M; 130 μL, 130 μmol) was added and the vial capped. The crude reaction mixture was heated at 150° C. for 15 min using a Smith Synthesiser microwave reactor. On cooling the vial was opened and the contents were filtered through a Whatman 5 μM filter tube, washing the filter cake with methanol (2×1 mL). The filtrate was evaporated and the resulting residue was purified using mass directed auto-preparative reverse phase HPLC to give the title compound (1.1 mg, 5%) as a white solid. LC / MS: 2.94 min; z / e 329, calcd (M−1) 329. 1H NMR (400 MHz: MeOD): 7.80 (4H), 7.75 (2H), 7.65 (2H), 4.45 (...
example 2
[0076]
{(Methylsulfonyl)[4′-(trifluoromethoxy)-1,1′-biphenyl-4-yl]amino}acetic acid
[0077]Prepared analogously to Example 1. LC / MS: 3.41 min; z / e 407, calcd (M+18) 407.
example 3
[0078]
[(4′-Acetyl-1,1′-biphenyl-4-yl)(methylsulfonyl)amino]acetic acid
[0079]Prepared analogously to Example 1. LC / MS: 2.98 min; z / e 365, calcd (M+18) 365.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| polar | aaaaa | aaaaa |
| wetting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com