Resonance tuning module for implantable devices and leads
a technology of resonance tuning and implantable devices, which is applied in the field of medical devices, can solve the problems of poor functionality of the pacemaker, the inability of the implanted device to be fully functional, and the inability to achieve the desired effect of the pacemaker,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
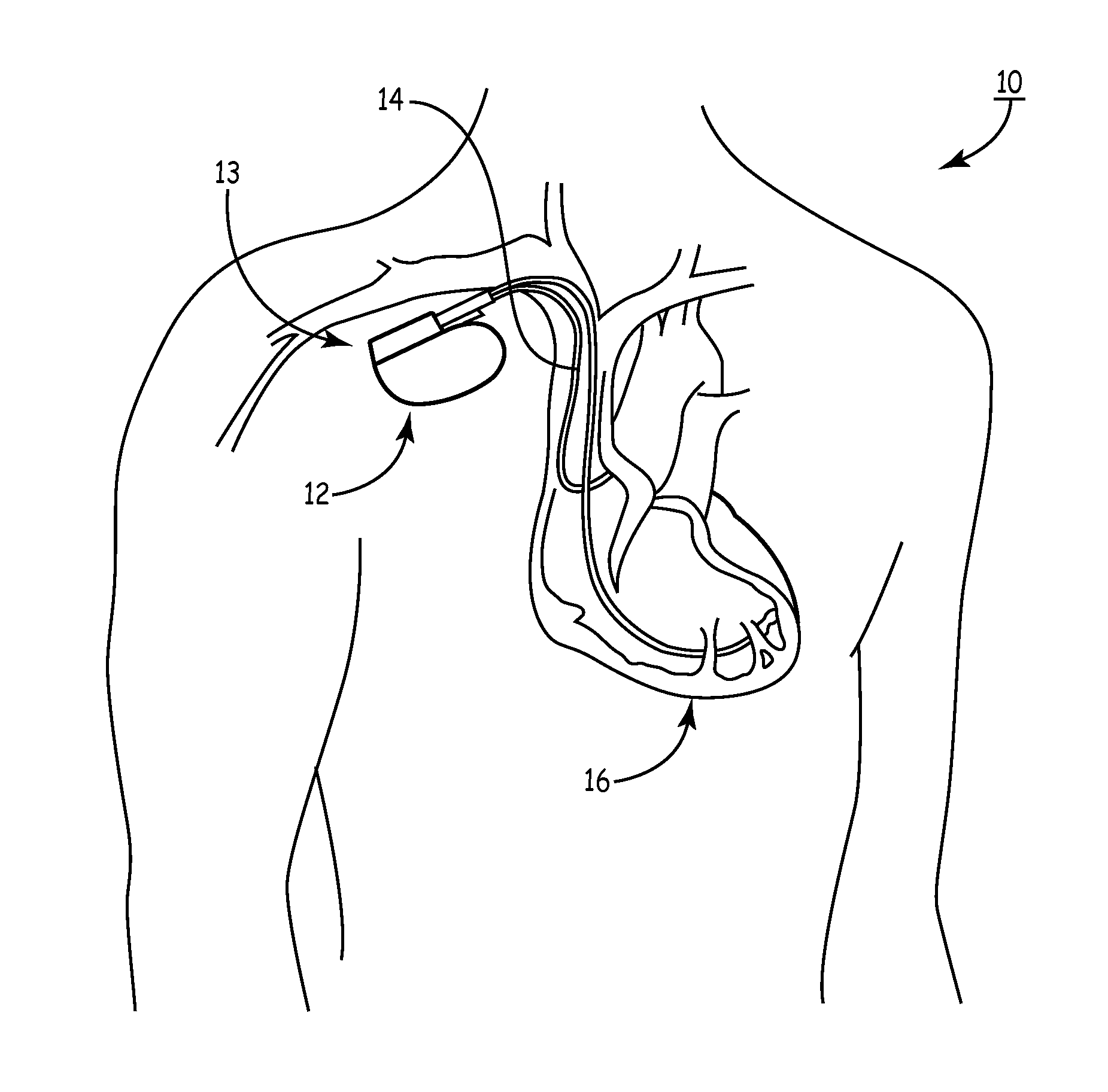
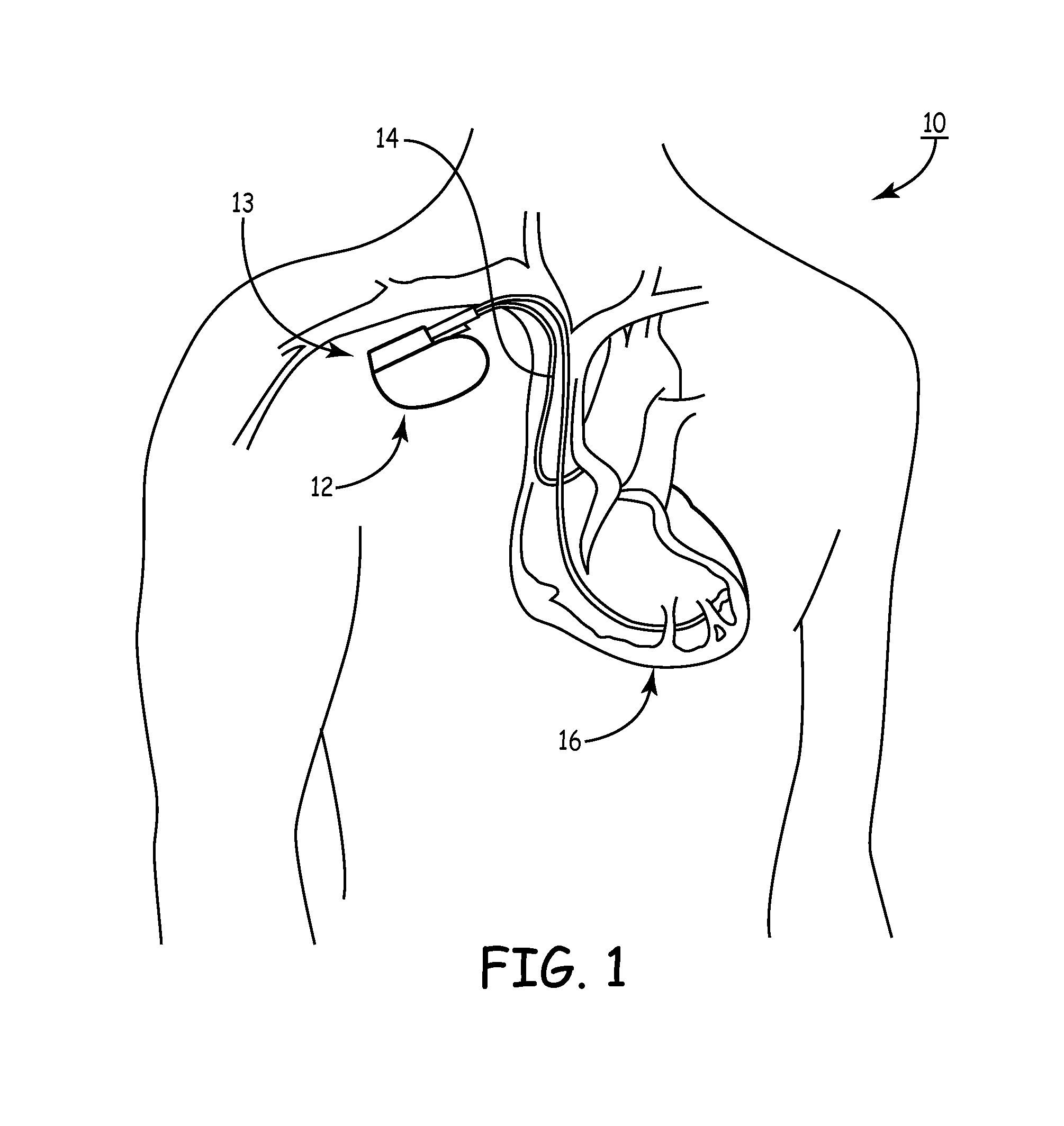
[0081]As noted above, a medical device includes an anti-antenna device to prevent or significantly reduce damaging heat, created by currents or voltages induced by outside electromagnetic energy (namely magnetic-resonance imaging), to a tissue area.
[0082]More specifically, the present invention is directed to a medical device that includes anti-antenna device, which significantly reduces the induced current on the “signal” wire of a pacing lead when the pacing lead is subjected to the excitation signal's frequency of a magnetic-resonance imaging scanner without significantly altering a low frequency pacing signal. The low frequency pacing signal may be generated by an implantable pulse generator or other pulse generator source outside the body.
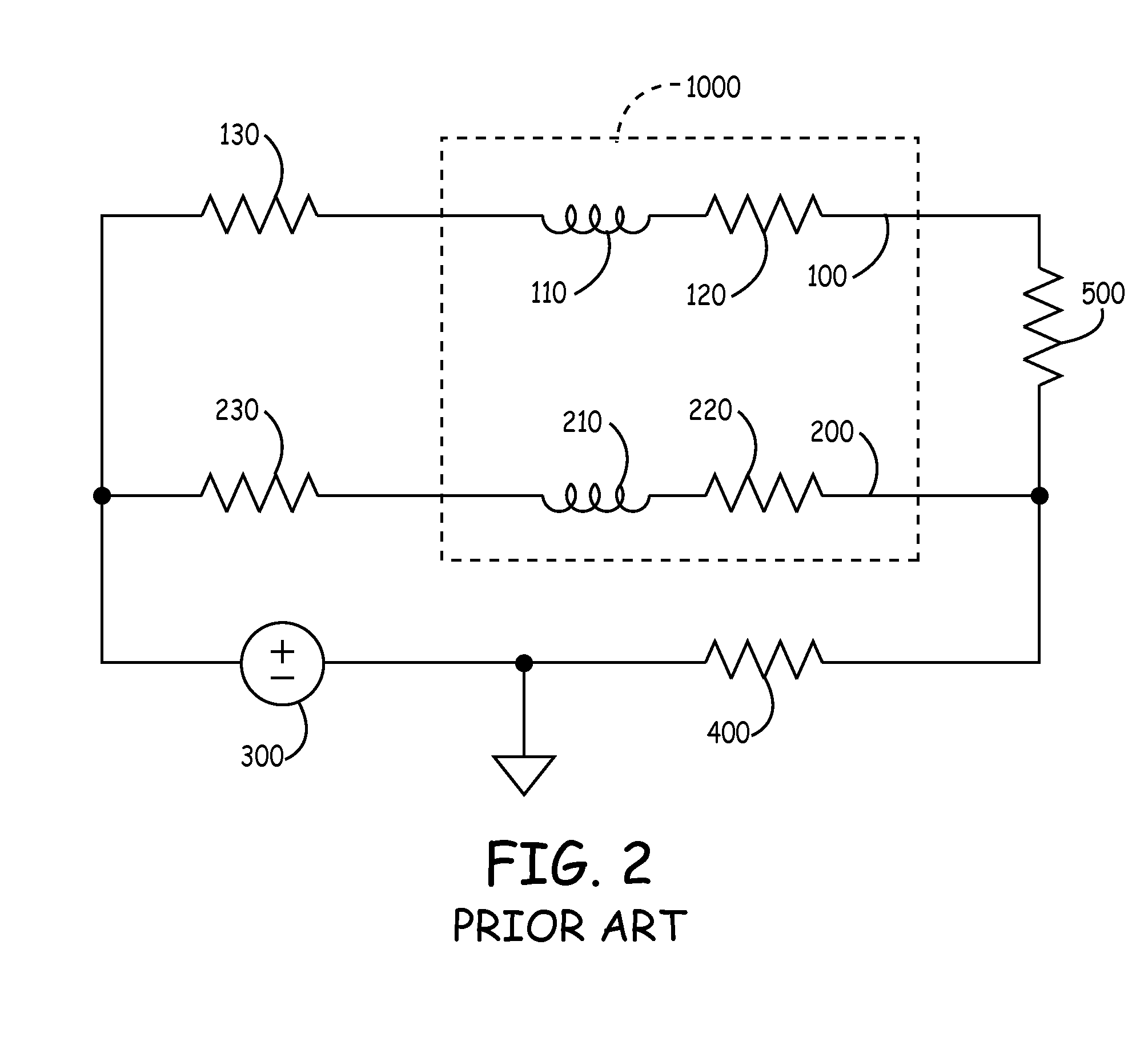
[0083]To provide an anti-antenna device, in one embodiment of the present invention utilizes a resonant circuit or circuits in line with a lead. The lead may be a signal wire of the pacing lead. Although the following descriptions of the vario...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com