Article of manufacture comprising aromatic amino acids isomers, analogs, or derivatives thereof to treat neurological disorders involving dysfunction of glutamatergic synaptic transmission
a technology of glutamatergic synaptic transmission and aromatic amino acids, which is applied in the direction of peptide/protein ingredients, heterocyclic compound active ingredients, biocide, etc., can solve the problems of limited use of current glutamate receptor modulators, limited clinical use of orally administered tyrosine, and limited use of cocaine dependence patients. , to achieve the effect of modulating glur activity, inhibiting glur activity, and lowering glu concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Effect of Aromatic Amino Acids (AAAs) on Miniature Excitatory Postsynaptic Currents (mEPSCs) in Hippocampal Neurons
[0066] Hippocampi were dissected from newborn rats and treated with trypsin to dissociate the cells. The hippocampal cells were then resuspended in Neurobasal Medium containing B-27 serum-free supplement, and cultured. Various concentrations of L-Phe, L-Tyr, L-Trp, dizocilpine (MK-801; (+)-5-methyl-10,11-dihydroxy-5H-dibenzo(a,b)cyclohepten-5,10-imine) were added to the extracellular solution. Electrophysiological recordings of miniature excitatory postsynaptic currents (mEPSCs) in cultured rat hippocampal neurons were made in the whole cell configuration. The current data was then digitized and analyzed.
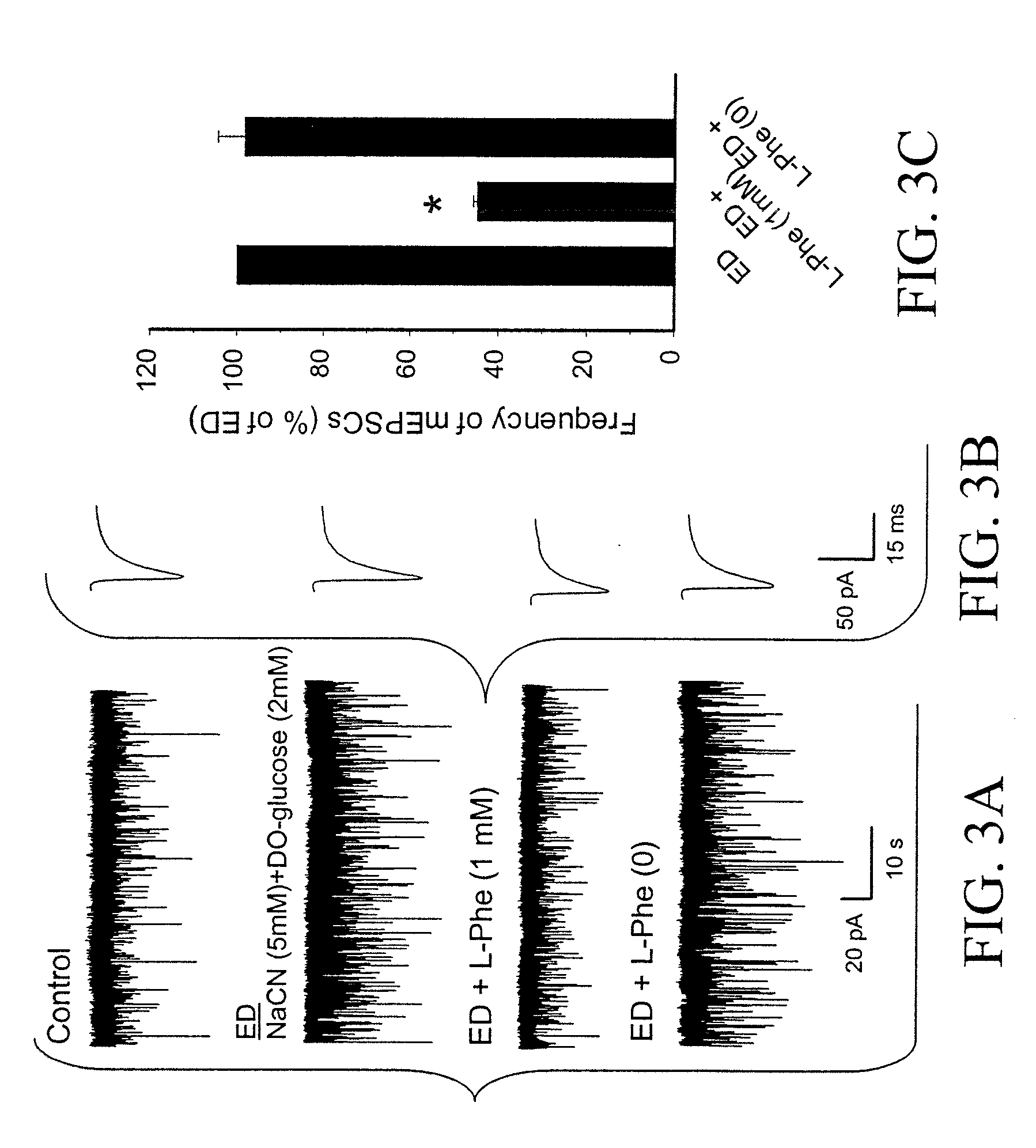
[0067]FIGS. 1A-1C show the effect of L-Phe on mEPSCs in rat cultured hippocampal neurons. Examples of mEPSCs, recorded in the presence of the NMDA channel blocker MK-801 (10 M) before, during, and after application of L-Phe (0.1 mM, 0.3 mM, 1 mM, and 3 mM) are sho...
example 2
Evaluation of the Neuroprotective Effects of AAAs in Hippocampal Cell Cultures
[0071] Neurons will be subjected to oxygen glucose deprivation (OGD) at 14-16 days in vitro. Neurobasal medium (Gibco / Life Technologies, Calif.) will be removed and put aside. Glucose-free medium, warmed to 37° C., will be applied to the neurons. Cultures will then be placed into an airtight chamber (Billups-Rothenberg Inc., Del Mar, Calif.) flushed with 95% N2 / 5% CO2 until oxygen concentration fell to less than 1%. The chamber should be maintained at 37° C. for 1-2.5 hr, the original conditioned Neurobasal medium will be reapplied, with or without AAAs (1 mM) and will be returned to the incubator for the duration of the experiment.
[0072] Cell viability at different experimental conditions can be assessed by counting phase-bright cells (live cells) under phase-contrast microscopy and propidium iodide (PI)-labeled nuclei (dead cells) under fluorescence microscopy). Viability can be calculated as the ratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com