Analogs of discodermolide and dictyostatin-1, intermediates therefor and methods of synthesis thereof
a technology which is applied in the field of analogs of discodermolide and dictyostatin1, intermediates, can solve the problems of difficult synthesizing discodermolide, difficult to ensure a sufficient supply of discodermolide, and insufficient supply of discodermolid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
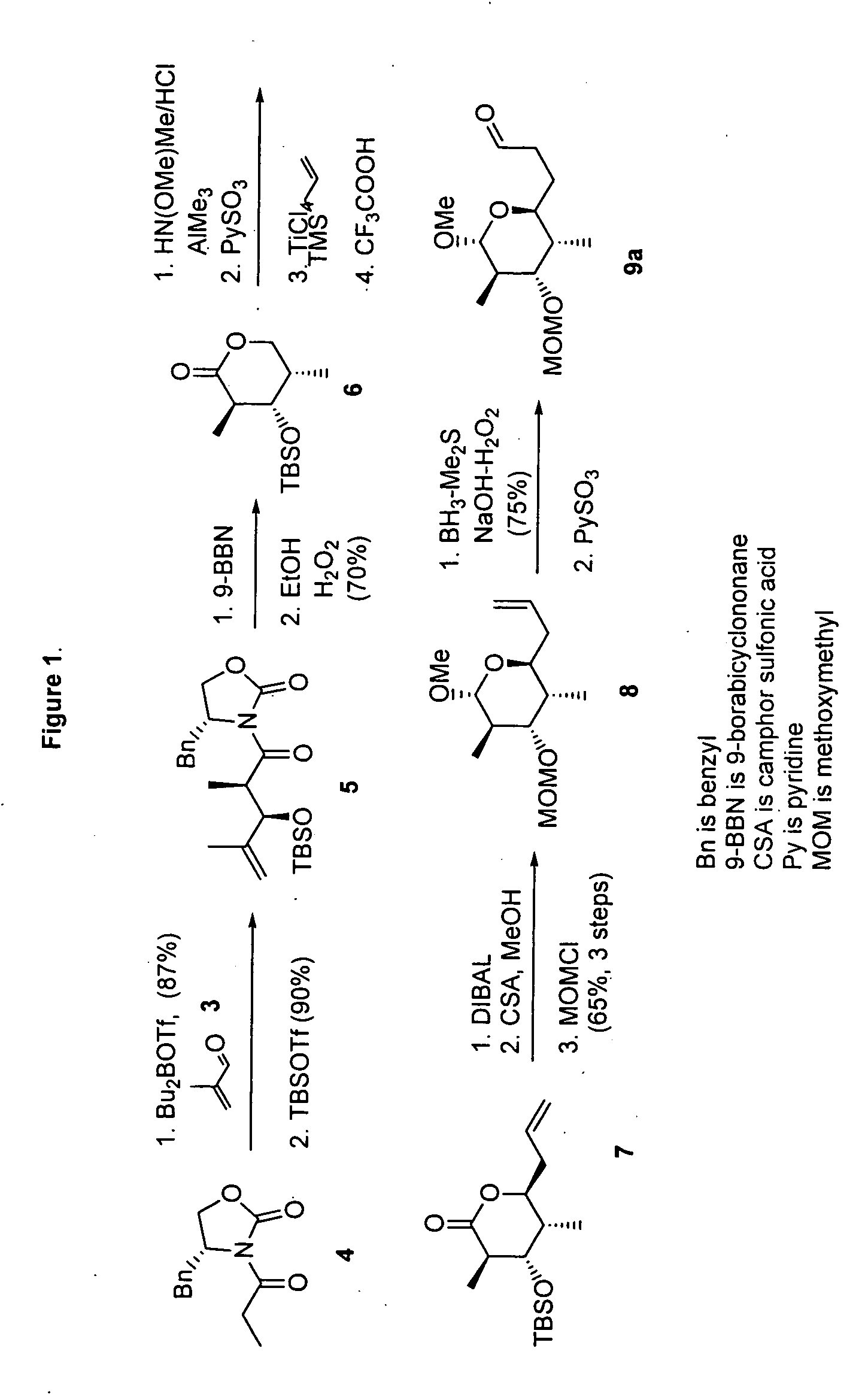
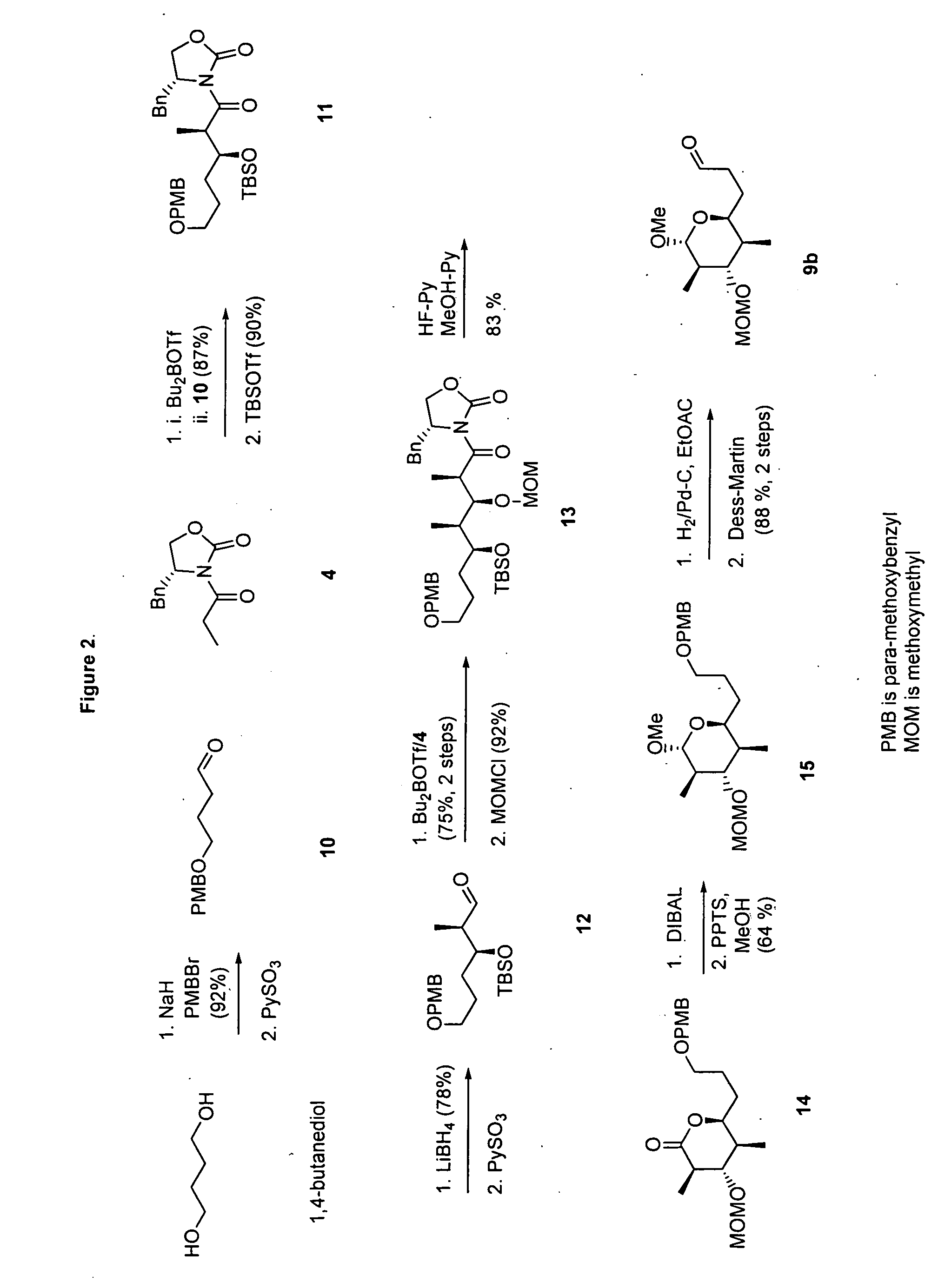
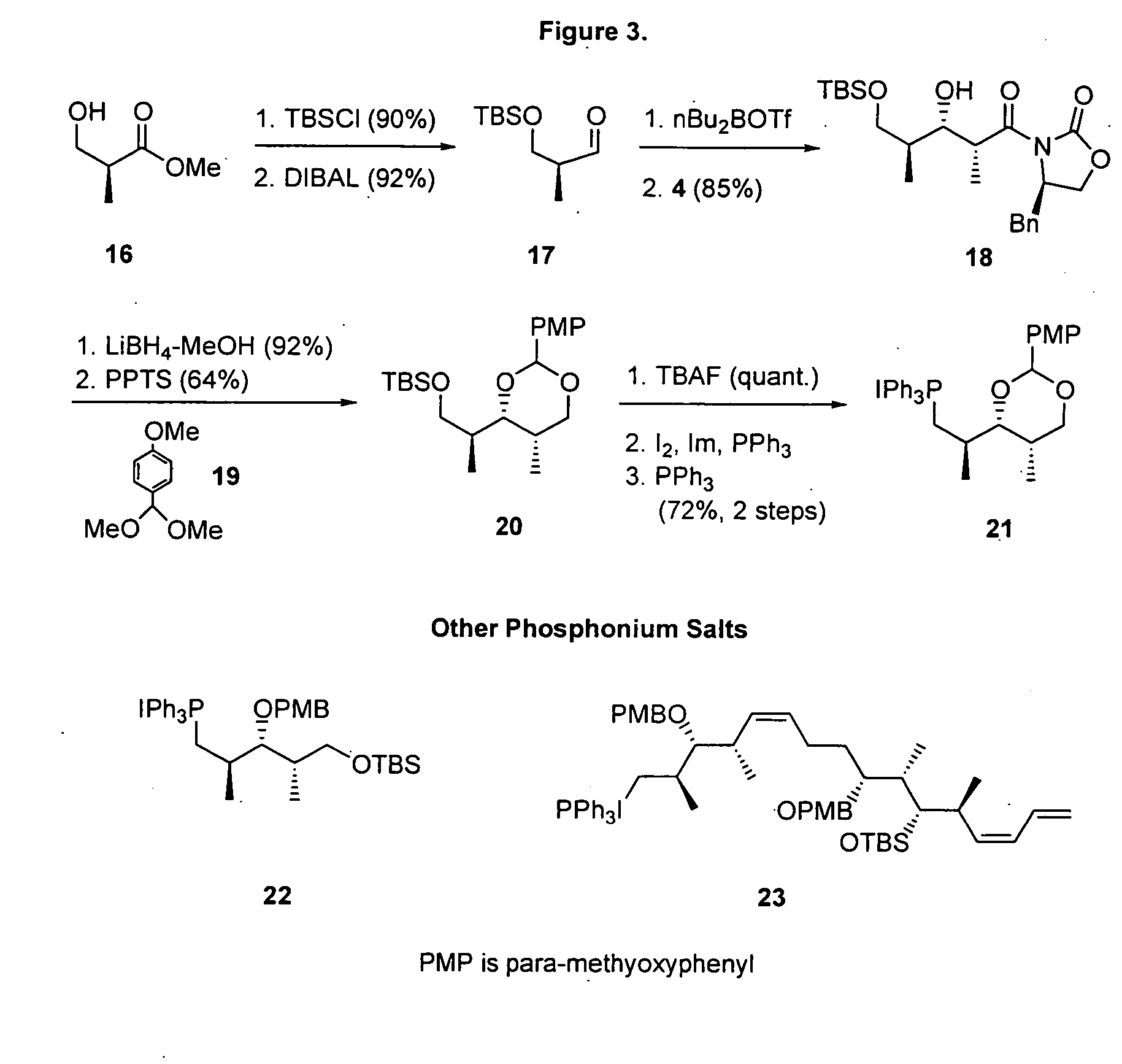
[0094] (4R)-4-Benzyl-3-[(2R,3R)-3-(tert-butyidimethylsilanyloxy)-2,4-dimethylpent-4-enoyl]oxazolidin-2-one (5). TBDMSOTf (3.44 mL, 15 mmol) was added to a stirred solution of aldol product (3.03 g, 10 mmol) and 1,6-lutidine (2.32 mL, 20 mmol) in CH2Cl2 (20 mL) at −78° C. and the mixture was stirred for 2 h at ambient temperature. The reaction was quenched by the addition of aqueous HCl (0.5 N, 50 mL). The resulting mixture was extracted with CH2Cl2 and dried over MgSO4 followed by the evaporation of solvent under reduced pressure. The product was purified by short column chromatography (hexane / EtOAc 9:1). Crude 5 was used without purification.
[0095] (2S,3R,4S,5R)-2-Allyl-6-methoxy-4-methoxymethoxy-3,5-dimethyltetrahydropyran (8). Diisobutylaluminum hydride (1.0 M in THF, 3.3 mL, 3.3 mmol) was added dropwise to a stirred solution of 7 (894 mg, 3 mmol) in anhydrous CH2Cl2 (30 mL) under an atmosphere of N2 at −78° C. and the resulting mixture was stirred for an additional 1 h at −78° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water soluble | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
| structure/activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com