Methods for cancer imaging
a cancer and imaging technology, applied in the direction of diagnostic recording/measuring, ultrasonic/sonic/infrasonic diagnostics, sugar derivates, etc., can solve the problems of many biopsy methods invasive, cancer is dangerously fatal, and many cancer deaths are still
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0123] This example illustrates the time course of uptake of 2-NBDG in Raji lymphoma cells.
Materials:
[0124] Raji cells from culture of one t-75 flask. Phosphate-buffered saline plus 2.5 mM CaCl2, 1.2 mM MgSO4, 4 mM KCl and 5 mM glucose (PBS+). 2-NBDG can be prepared in accordance with the method reported in Yoshioka et al., 1996, Biochimica et Biophysica Acta 1289: 5-9, incorporated herein by reference.
Uptake Reaction:
[0125] Culture medium with cells (15 mL) was centrifuged for 10 min at 800×g. The pellet of cells was resuspended in PBS+ and centrifuged for 10 min at 800×g, and the resulting pellet was resuspended in 1.5 mL PBS+. Aliquots (100 uL) were placed into each of 8 tubes and incubated at 37° C. for 15 min. The fluorophore deoxyglucose conjugate 2-NBDG was added to each tube to provide a concentration of 200 uM. A tube was removed from the 37° C. bath at each of 0, 1, 3, 5, 10, 20, 30 and 40 min and immediately placed on ice. The cells were washed twice with PBS and re...
example 2
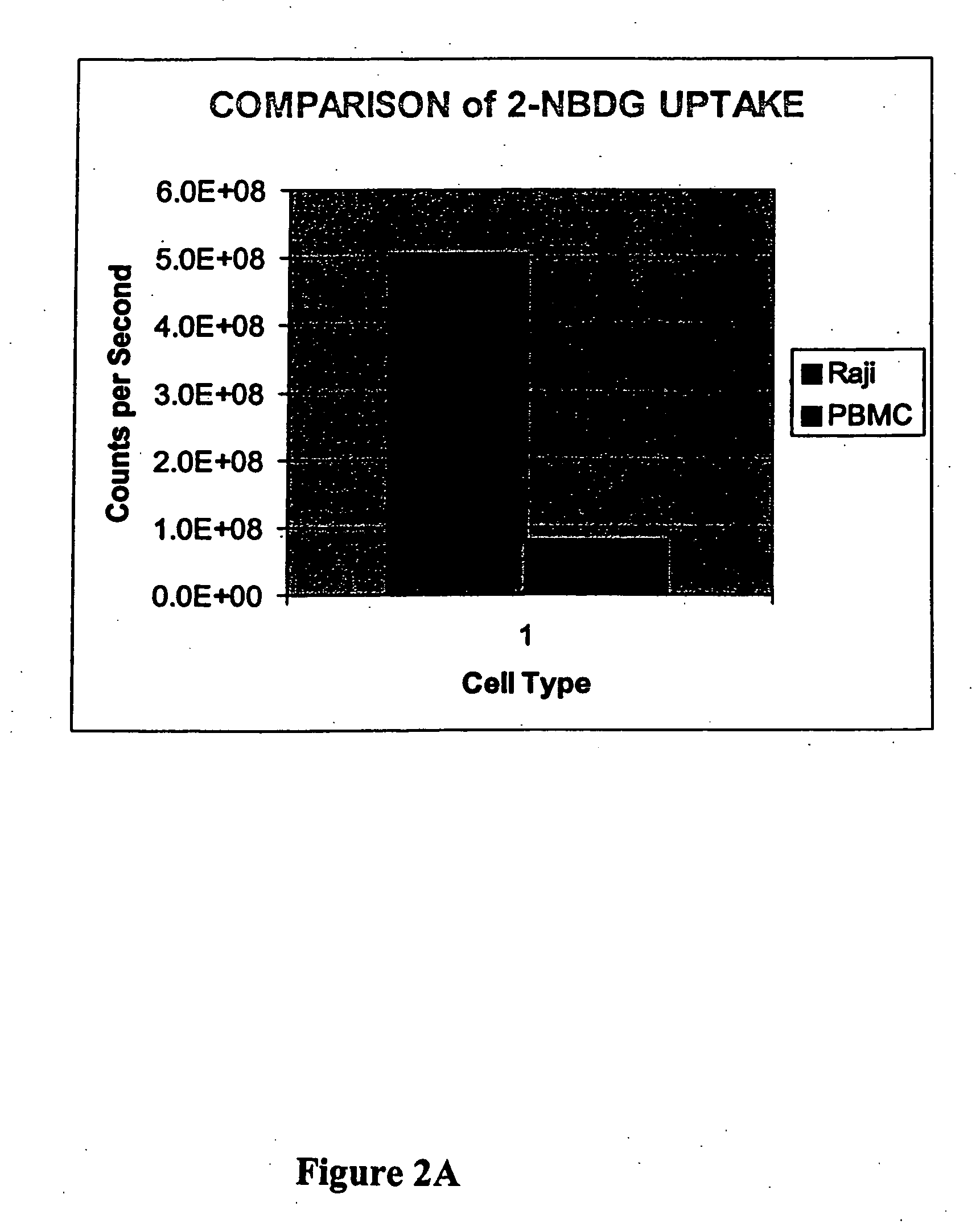
[0126] This example illustrates a comparison of 2-NBDG uptake in Raji lymphoma cells and peripheral blood white cells and illustrates the preferred uptake of a fluorophore deoxyglucose conjugate by a cancer cell as compared to a normal cell.
Materials:
[0127] Raji cells from culture one t-75 flask. Peripheral blood white cells from Stanford Blood Bank. Leukocytes were further separated by Ficoll separation (twice). These cells are referred to as PBMC peripheral blood mononuclear cells). Phosphate-buffered saline. 2-NBDG, 0.1 M in water.
Uptake Reaction:
[0128] Culture medium with cells (either Raji or PBMC, 15 mL) were centrifuged for 10 min at 800×g. The pellet of cells was resuspended in PBS and centrifuged for 10 min at 800×g, and the resulting pellet was resuspended in 1.5 mL PBS. Cells were counted and adjusted to 1×106 per mL for both. Aliquots (100 uL) were placed into each of 2 tubes for each cell type, and the tubes were incubated at 37° C. for 15 min. The fluorescent deo...
example 3
[0129] This example illustrates the preparation of the fluorophore deoxyglucose conjugate of the invention FGC-002. In the conjugate, the fluorophore is BODIPY, and the glucose derivative is glucosamine.
[0130] Generally, the FGC-002 conjugate is prepared by treating 6-((4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionyl)amino)hexanoic acid, succinimidyl ester (BODIPY from Molecular Probes, D-2184, MW 502) with an excess of D-glucosamine (Sigma) in an aprotic solvent with gentle heating. Isolation of the product (FGC-002) can be accomplished via chromatography.
[0131] More particularly, 2.2 mg (4 micromoles) of 6-((4,4-difluoro-5,7-diethyl-4-bora-3a,4a-diaza-s-indacene-3-propionyl)amino)hexanoic acid, succinimidyl ester was dissolved in 0.3 ml of DMF followed by the addition of 6 mg (28 micromoles) of glucosamine HCL dissolved in 0.3 ml of water and 3.9 microliters (28 micromoles) of triethyl amine. The reaction was stirred for 24 hrs at room temperature, evaporate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission wavelength | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com