Methods and apparatus for synthesis of metal hydrides
a metal hydride and apparatus technology, applied in the field of electrochemical reduction of active metal salt compounds, can solve the problem that none of these processes has been implemented in commercial practi
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0048] A schematic illustration of the reactions taking place in the process is provided in FIG. 2. The working electrode (cathode) is a nickel wire. The counter electrode (anode) is a platinum mesh inside a glass sparging tube. The interior of the glass sparger comprises the anode chamber, and the region external to the sparging tube comprises the cathode chamber.
[0049] A mixture consisting of about 39.2 g LiBr, 18.1 g KBr, and 42.8 g of CsBr was charged to cathode compartment and was electrolyzed at about 5 V for about 5 hours under a hydrogen atmosphere to produce lithium metal at the cathode and bromine at the anode. The tube impeded mixing of the bromine that formed at the anode with the melt external to the tube, and thus slowed the back-reaction of lithium and bromine to lithium bromide. The tube also facilitated removal of gaseous bromine from the reactor under a stream of flowing nitrogen. The reaction flask containing the melt was maintained in a constant temperature bath...
example 2
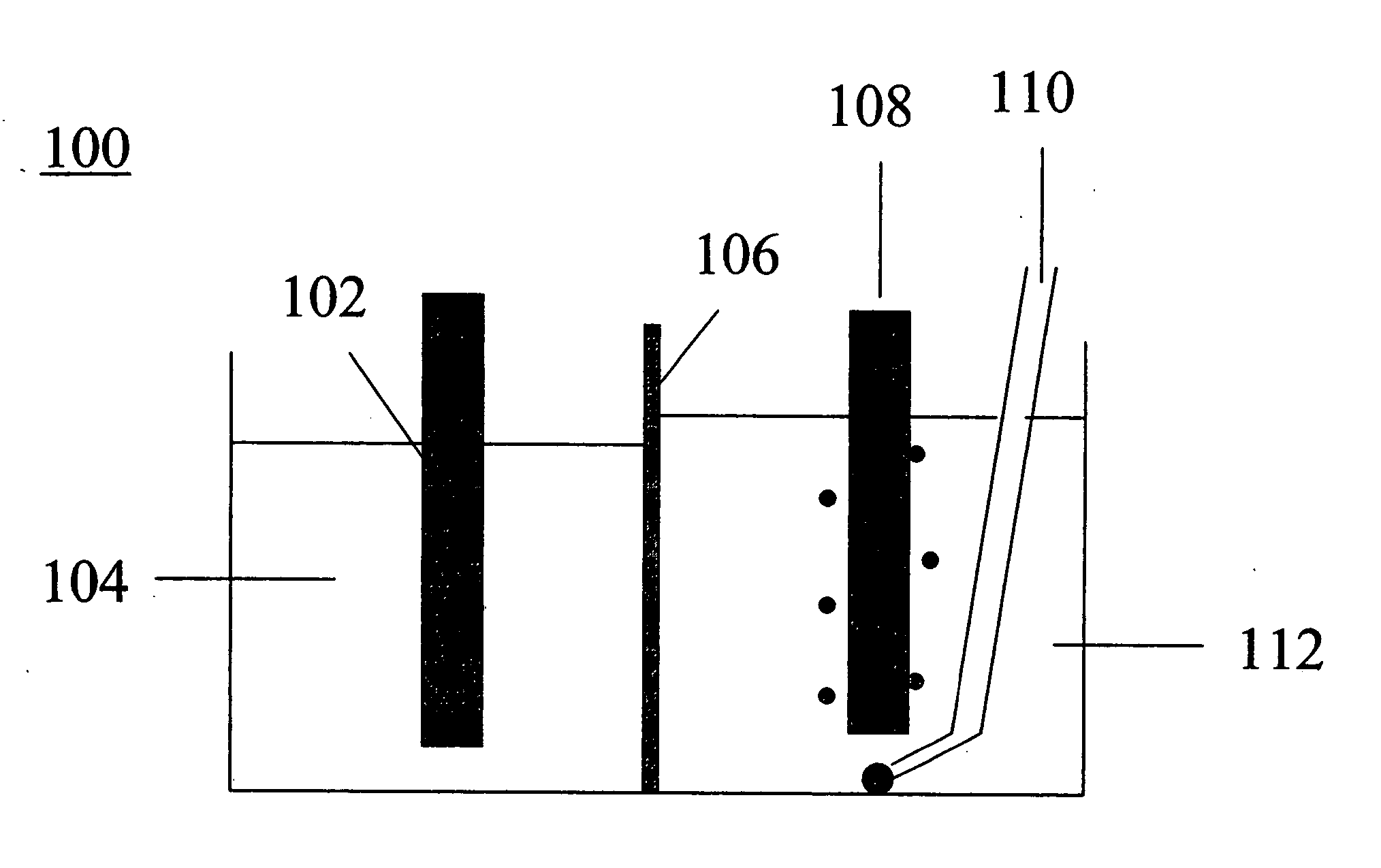
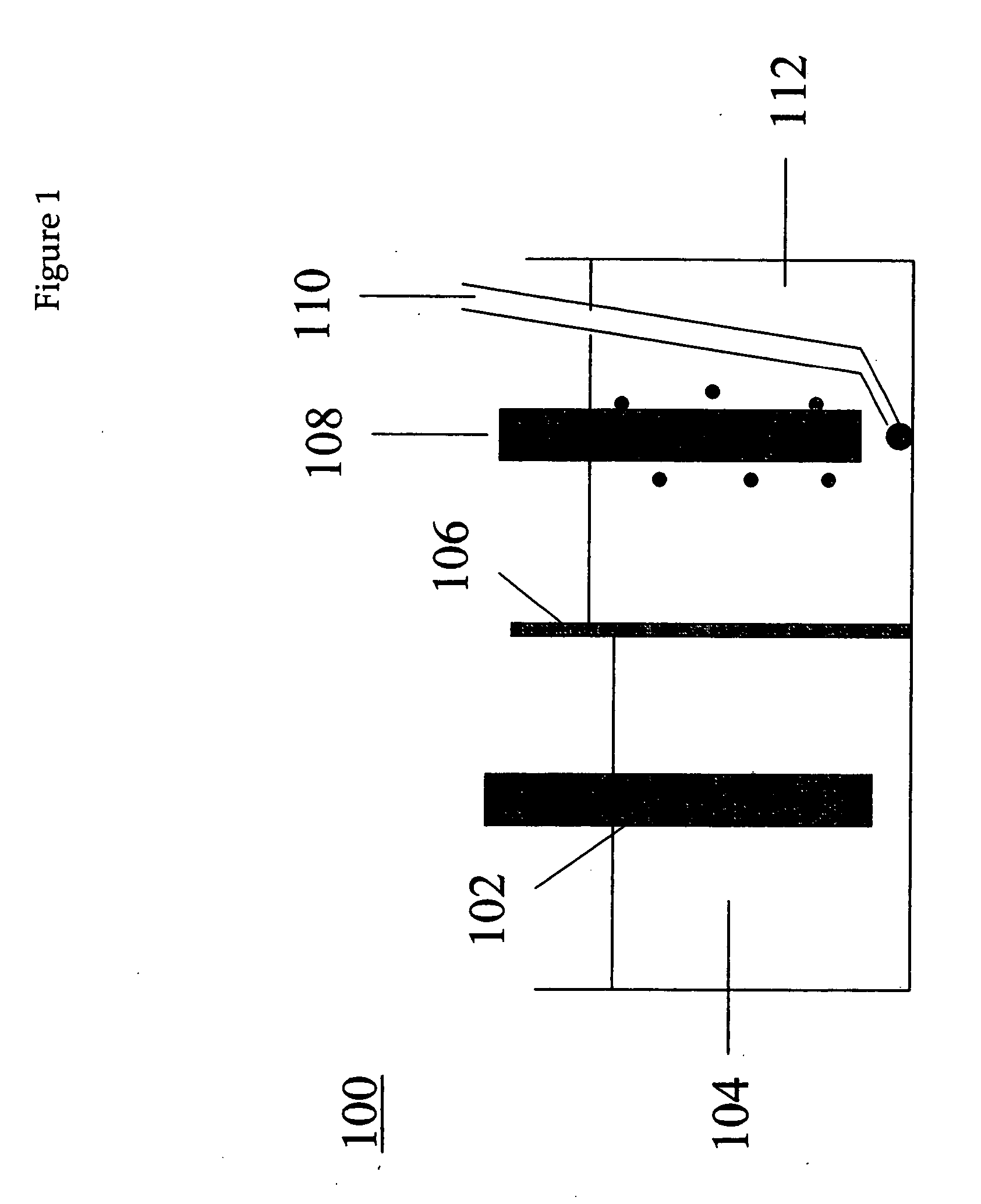
[0051] Using the procedure described in Example 1, a melt consisting of about 39.2 g LiBr, 18.1 g KBr, and 42.8 g of CsBr was electrolyzed under an argon atmosphere at about 3 V for 34 minutes. The potential, at 3 V, was too low to reduce the cations in the melt to metal, and instead reduced the Ni surface of the cathode and generated bromine at the anode. The reactor 400 was assembled as shown in FIG. 4. The reaction flask 402 containing the melt in cathode compartment 414 was maintained in a constant temperature bath at about 275° C. The working electrode (cathode) was a nickel frit 404 connected to an inlet 406 through which a gas was passed (the gas could exit the reactor via outlet 420). The counter electrode 408 (anode) was a platinum mesh inside a glass sparging tube 410 with a glass frit separator 412. After 34 minutes, argon flowing through the cathode frit was replaced by flowing hydrogen. Argon continued to flow over the anode to remove bromine. The current was not interr...
example 3
[0053] A melt consisting of about 9.8 g LiBr, 4.5 g KBr, and 10.7 g of CsBr under a nitrogen atmosphere was heated to about 250° C. To this melt, 1.6 grams of B2O3 was added. With stirring, 0.27 g of LiH was added to the melt. After adding LiH, the temperature bath was turned off, but stirring was continued until melt solidified. After dissolving the cooled melt in 100 mL of 0.5 M NaOH, a 50 mL sample of the solution was titrated using the iodate method for borohydride. The titration indicated that 5.3×10−3 mol BH4− was formed, a yield of 62% based on the 0.27 grams of LiH added to the reactor. Boron NMR of the aqueous solution confirmed the presence of the borohydride anion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com