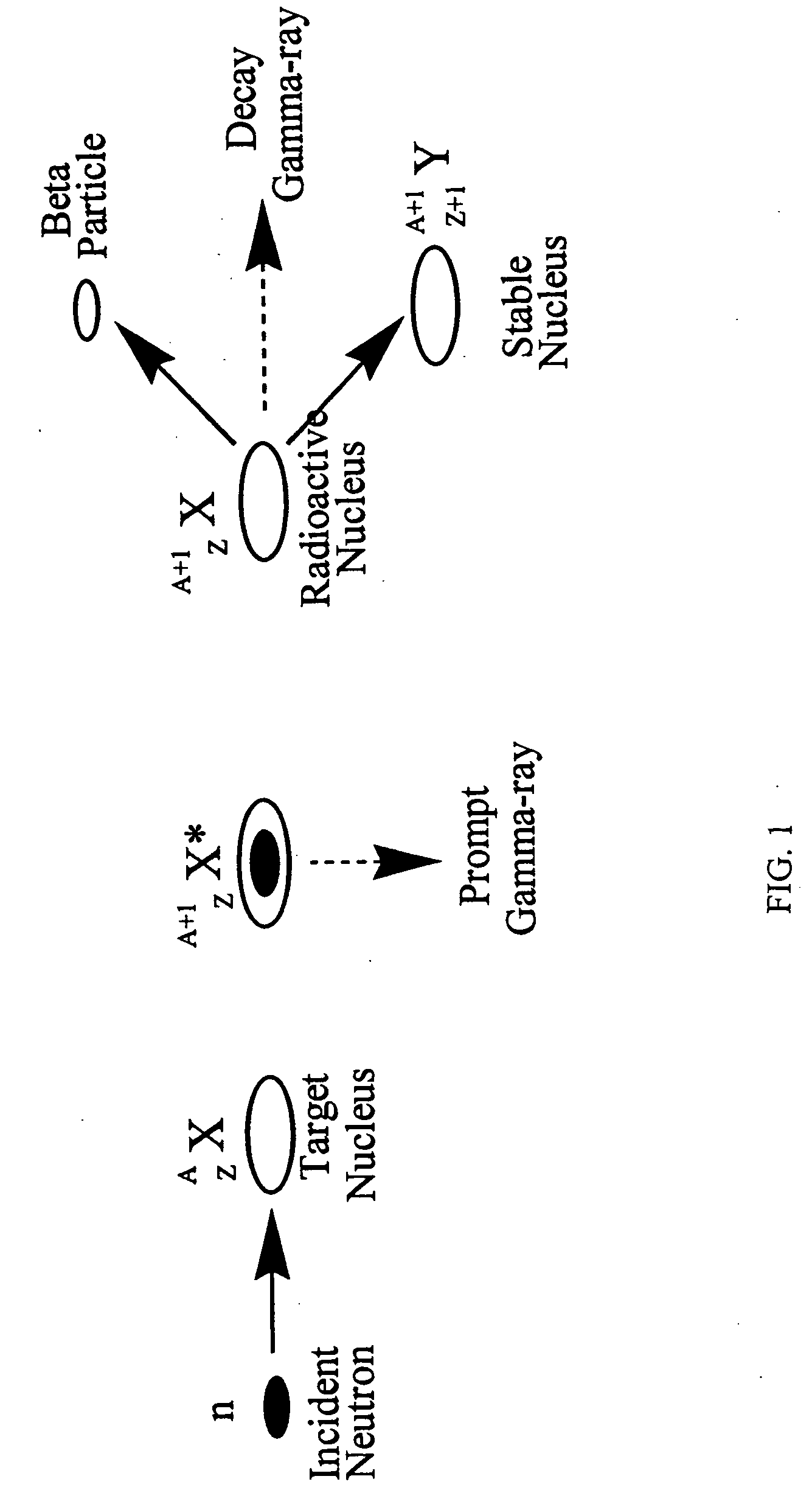
Synthesis, compositions and methods for the measurement of the concentration of stable-isotope labeled compounds in life forms and life form excretory products
a technology of stable isotope and excretory products, applied in the direction of heavy metal active ingredients, instruments, therapies, etc., can solve the problems that the present methods for detecting biological compounds or physiological processes of interest lack the requisite sensitivity and non-toxicity for in vivo administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
General Procedure of Measuring Blood Clearance
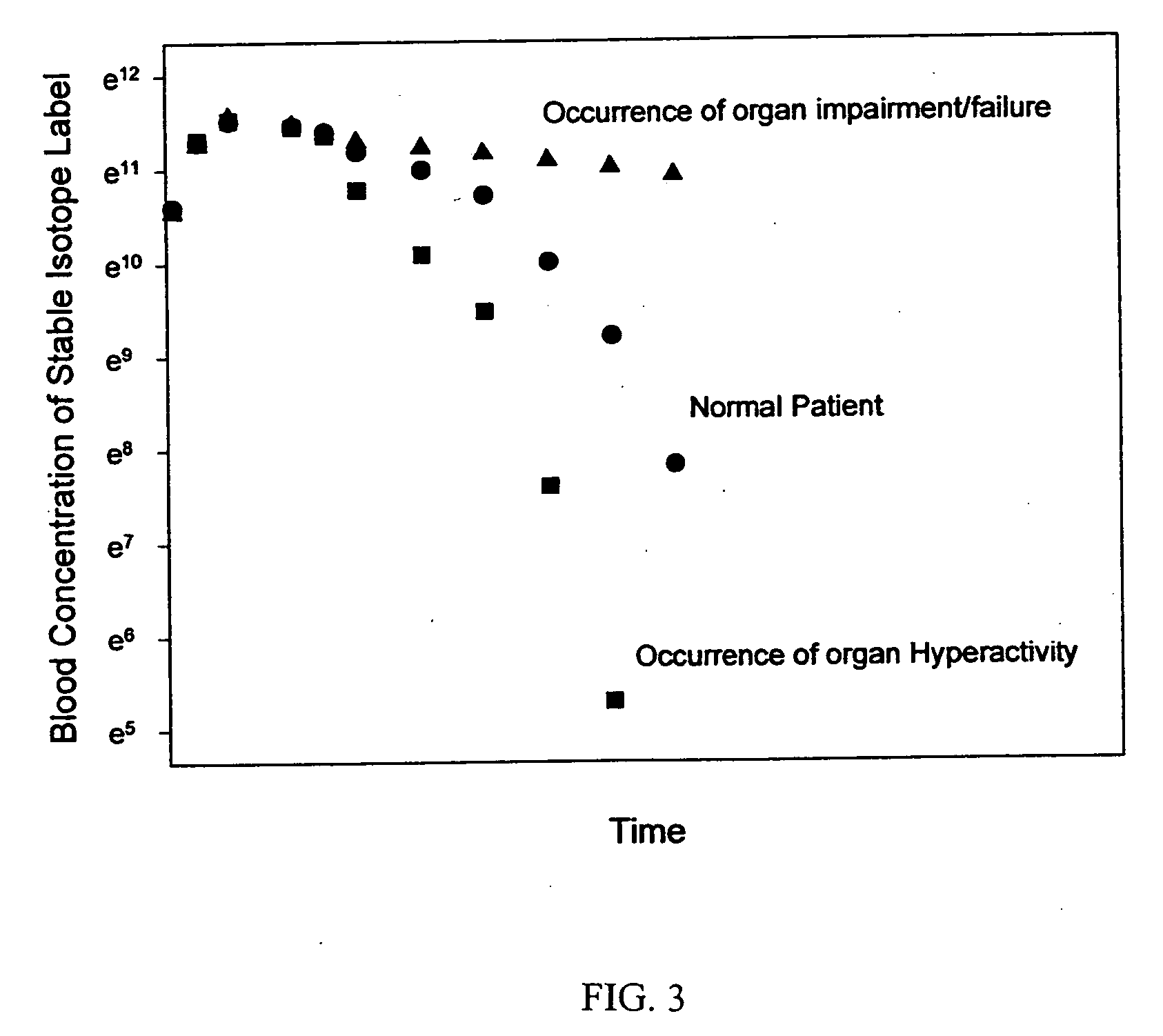
[0188] The method of the invention may be used to monitor the blood clearance mechanisms mediated by, for example, liver receptors and kidney function of a subject / patient and thereby provide information regarding the functioning of these mechanisms. Test substances designed to assess organ function, each labeled with a stable isotope may be injected into a subject either as a bolus or a constant infusion. In general, the expected relationships between test substance blood concentration as a function of time are shown in FIG. 3 (bolus injection) and 4 (constant infusion). FIG. 3 illustrates that, following a bolus injection of the test compound, the blood concentration of the test compound increases sharply, and then decreases over time until reaching its preinjection concentration in a normal subject. When organ impairment (e.g. kidney failure) occurs, the blood concentration of the test compound will decrease more slowly and take a l...
example 1
Post Labeling Sample Tubes with Monitor (Use of an Internal Standard to Correct for Neutron Flux Variation Between Samples)
[0207] This example illustrates the procedure for the use of an internal monitor to correct for neutron flux variation between sample tubes.
[0208] Tubes containing samples of samarium (10 μg), lutetium (10 μg), gold (10 μg) and arsenic (1 μg) for neutron activation were prepared by pipetting 100 μl of an aqueous solution of samarium, lutetium, and gold chloride and 25 μl of lithium arsenate-hexafluride into the bottom of each tube. The water was removed by heating the tubes at 70° C. for 12 hours. The tubes were exposed to a 2 megawatt neutron flux for 15 minutes. Five days later the tubes were counted for three minutes. Column 1 and 2 of Table 3 show the disintegrations per minute (dpm) and the correction factor calculated for the arsenic monitor. Since identical amounts of arsenic were added to each tube, all counts should be identical except for variation i...
example 2
Effect of Placement of Monitor in Various Locations within the Sample Tube when Used to Correct for Sample Count Rate
[0209] This example illustrates the importance of locating neutron flux monitor in identical portions within each tube, and counting each tube with the same geometry.
[0210] A sample of 100 μl of arsenic monitor containing, for example, 10 μg As is added to each of three tubes, at the bottom of the tube (tube 1), the middle of the tube (tube 2), or in the cap of the tube (tube 3). The water is removed by heating at 70° C. allowing the monitor to be deposited as a dry film. Next, 100 μl of samarium (10 ng) is added to the bottom of each tube without disturbing the monitor and the water is removed by heating. Samarium and arsenic solutions are prepared as in Example 1. The three tubes are taped together and exposed to a 1 megawatt neutron flux for 15 minutes. Since the sample tubes are close enough together to assure that neutron flux is experimentally constant across ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com