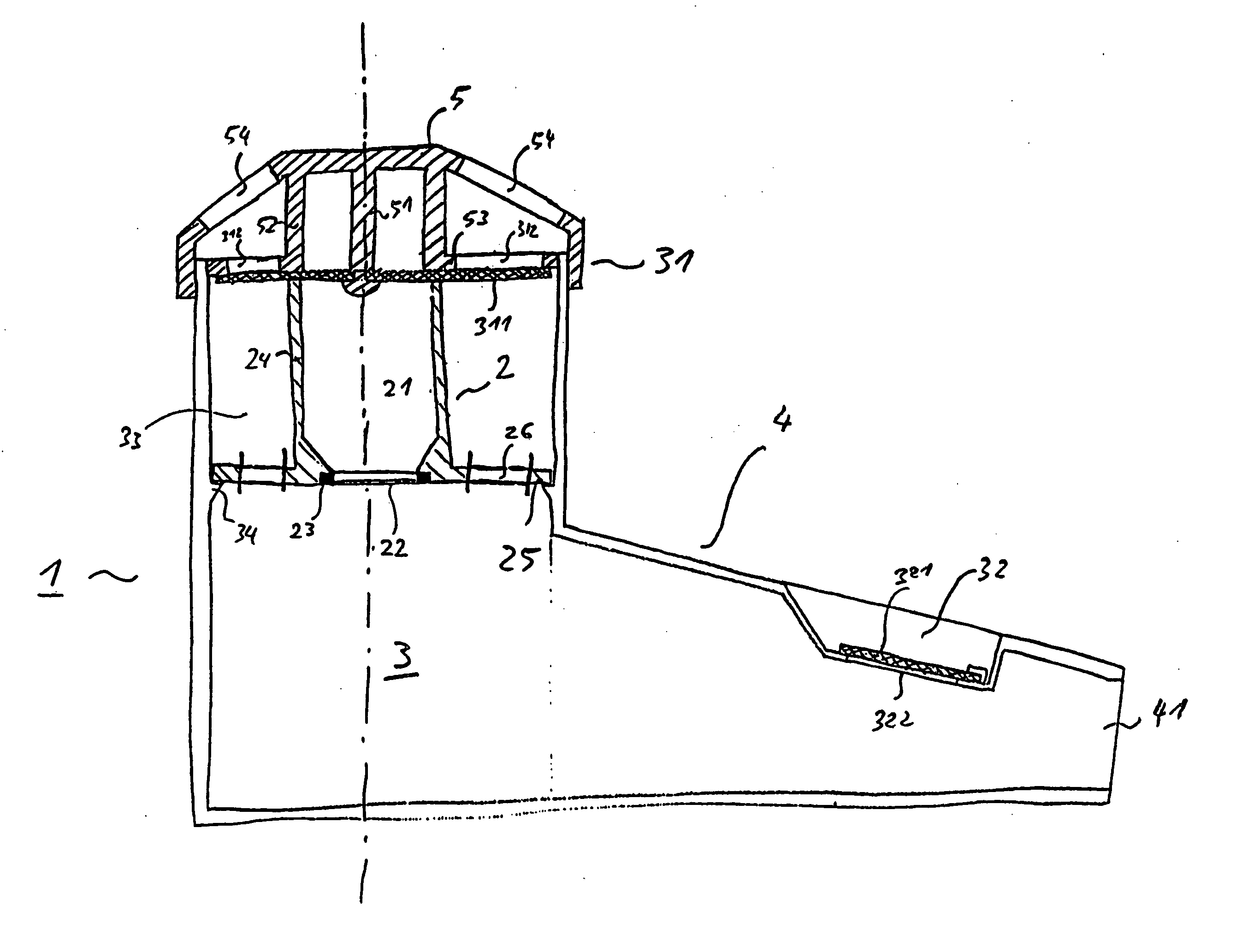
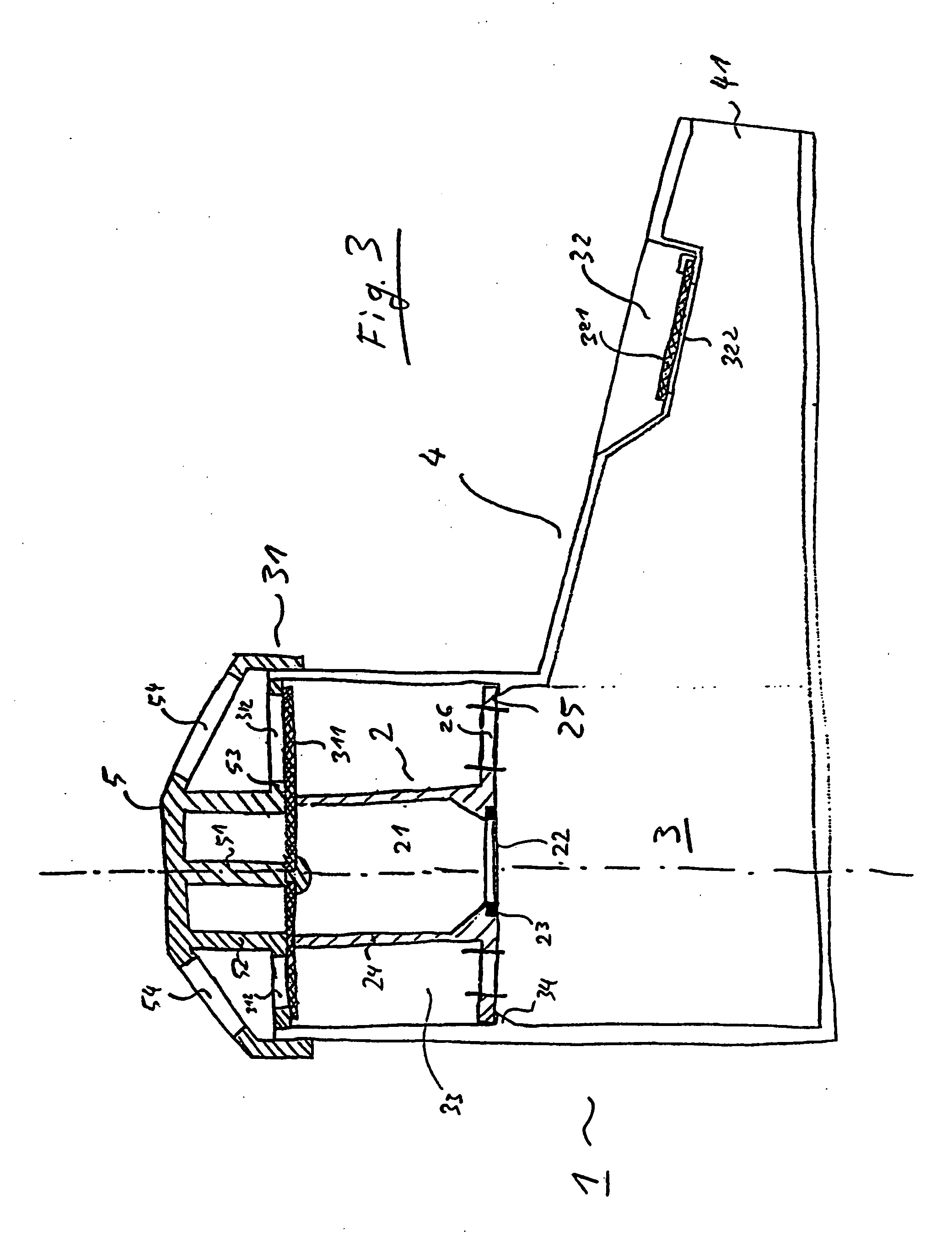
Inhalation nebulizer
a nebulizer and inhalation technology, applied in the direction of medical atomisers, spraying apparatuses, liquid spraying apparatuses, etc., can solve the problem of enormous technical expenditure of automatic control of aerosol production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working example 1
[0083] The inhalation nebulizer described herein was used to test the atomization of two commercial products along with a novel submicron suspension. A salbutamol-sulfate solution available commercially as VENTOLIN™ (known generically as albuterol) was tested, as was a budesonide suspension sold under the name PULMICORT™. For comparison purposes, a novel submicron suspension of budesonide was also tested. Each solution or suspension was tested during continuous mode and during breath-triggered mode to evaluate the performance of the inhalation nebulizer.
[0084] Evaluation included looking at the percent of the test solution available for capture by an inhalation filter, as well as looking at product that remained in the mixing chamber, the fluid feed, or was otherwise lost or unrecoverable.
[0085] The results are depicted graphically in FIG. 4. The results indicate that deposition to an inhalation filter can be increased from about 50 percent to about 75 percent by operating in the ...
working example 2
[0086] The inhalation nebulizer described herein was tested against a commercially available product (the PARI LC STAR) using the three test components described in the previous Example. FIG. 5 provides results for the LC STAR and the results achieved by the inventive nebulizer (PEN). The results clearly indicate that the nebulizer in accordance with the invention provides for a higher proportion of drug available at smaller average particle sizes. Specifically, the MMAD is shifted from about 3.7 to 5.1 μm (for the PARI LC STAR) to about 2.2 to 3.9 μm for the inventive nebulizer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com