Chimeric proteins for diagnosis and treatment of diabetes
a technology of chimeric proteins and diabetes, applied in the direction of lyase, hormone peptides, peptide/protein ingredients, etc., can solve the problems of multiple organ system complications, high blood glucose levels, hyperglycemia, etc., to facilitate diagnosis and prognostic evaluation, and enhance beneficial effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Genes for Expression of Chimeric Proteins of the Invention
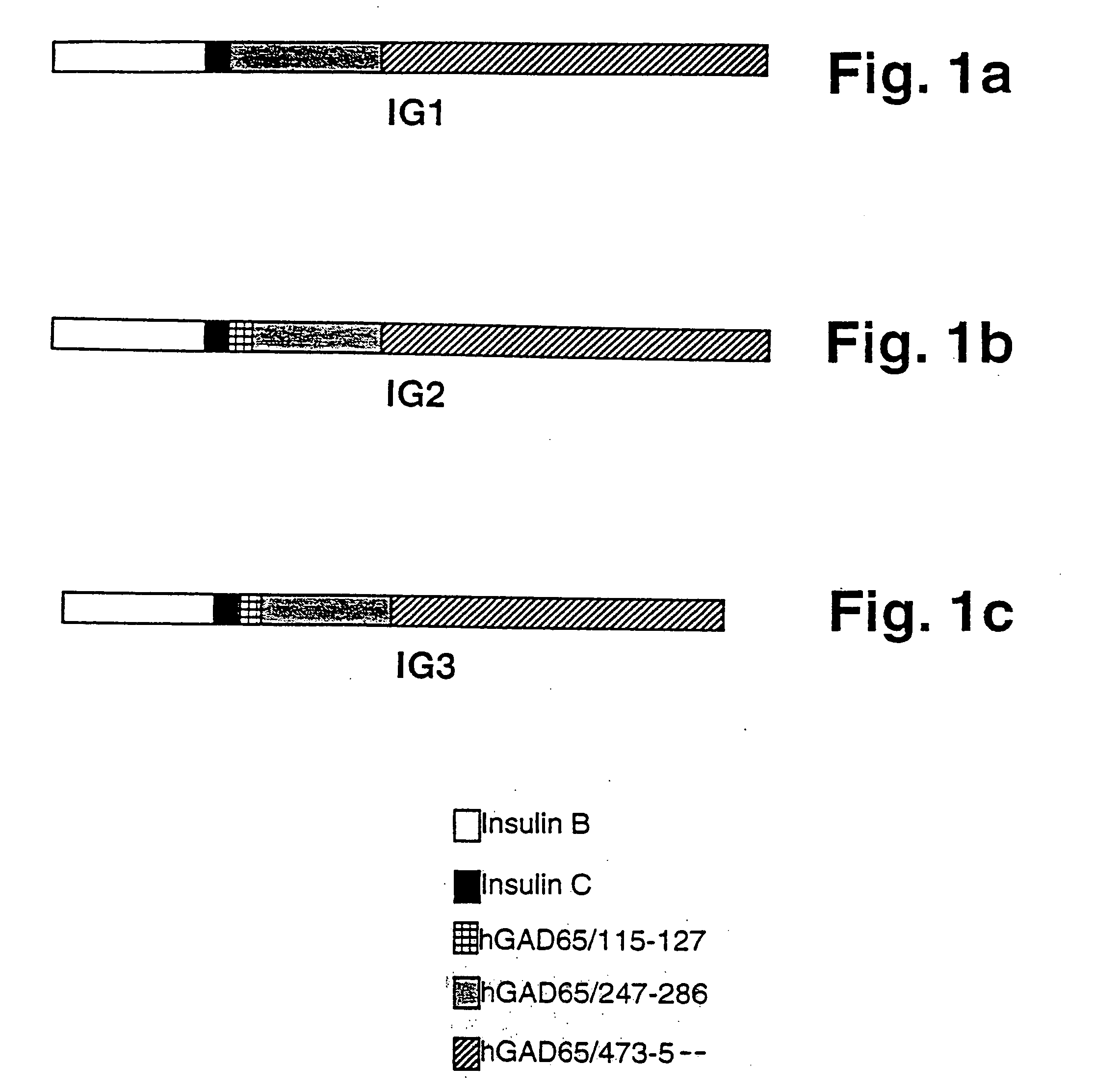
[0102] IG1. A synthetic chimeric gene encoding chimeric protein IG1 (SEQ ID NO:1) was constructed in two rounds of overlapping PCR (Ho et al., 1989). Each gene subdomain was synthesized in a standard PCR 100 μl reaction using 10 pmole of each of the appropriate oligonucleotide primers, as described below. The 5′ gene segment was amplified using the overlapping primers:prIG1 (SEQ ID NO:11), and prIG2 (SEQ ID NO:12). The 3′ gene segment was amplified using the overlapping primers prIG3 (SEQ ID NO:13) and prIG4 (SEQ ID NO:14). The prIG1 oligo-nucleotide (SEQ ID NO:11) was designed to allow creation of a unique NdeI restriction site at the 5′ end. The prIG4 primer (SEQ ID NO:14) included a unique BamHI site, stop codon (TAA) and 18 nucleotides that encoded a histidine tag sequence for purification of the recombinant chimeric protein by metal affinity chromatography. The two subdomains were combined using flanking oligonucleotid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com