Method and apparatus for filling a cavity
a cavity and filling technology, applied in the field of non-soft tissue cavity implants, implant applicators, delivery devices, etc., can solve the problems of unhealthy pressure on other parts of the body, change in the center of gravity of the body, permanent discomfort, etc., to achieve sufficient crush strength, sufficient material strength, and stable body region
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0139] In general, the implants and / or applicators described herein may be used to distract an existing body region. In one version, the body region is a non-soft tissue cavity. In one version, the body region is a hard tissue cavity, such as a bone cavity arising from a tumor, injury or surgery.
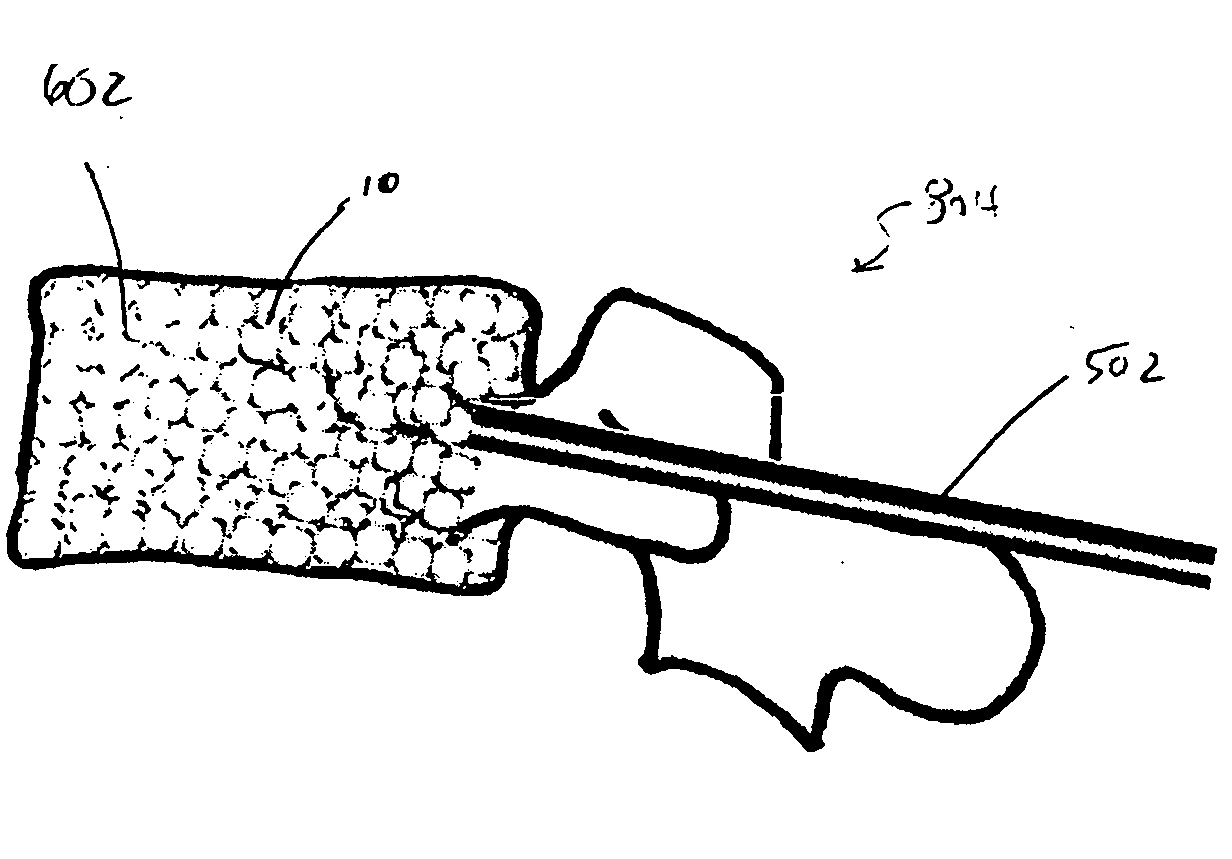
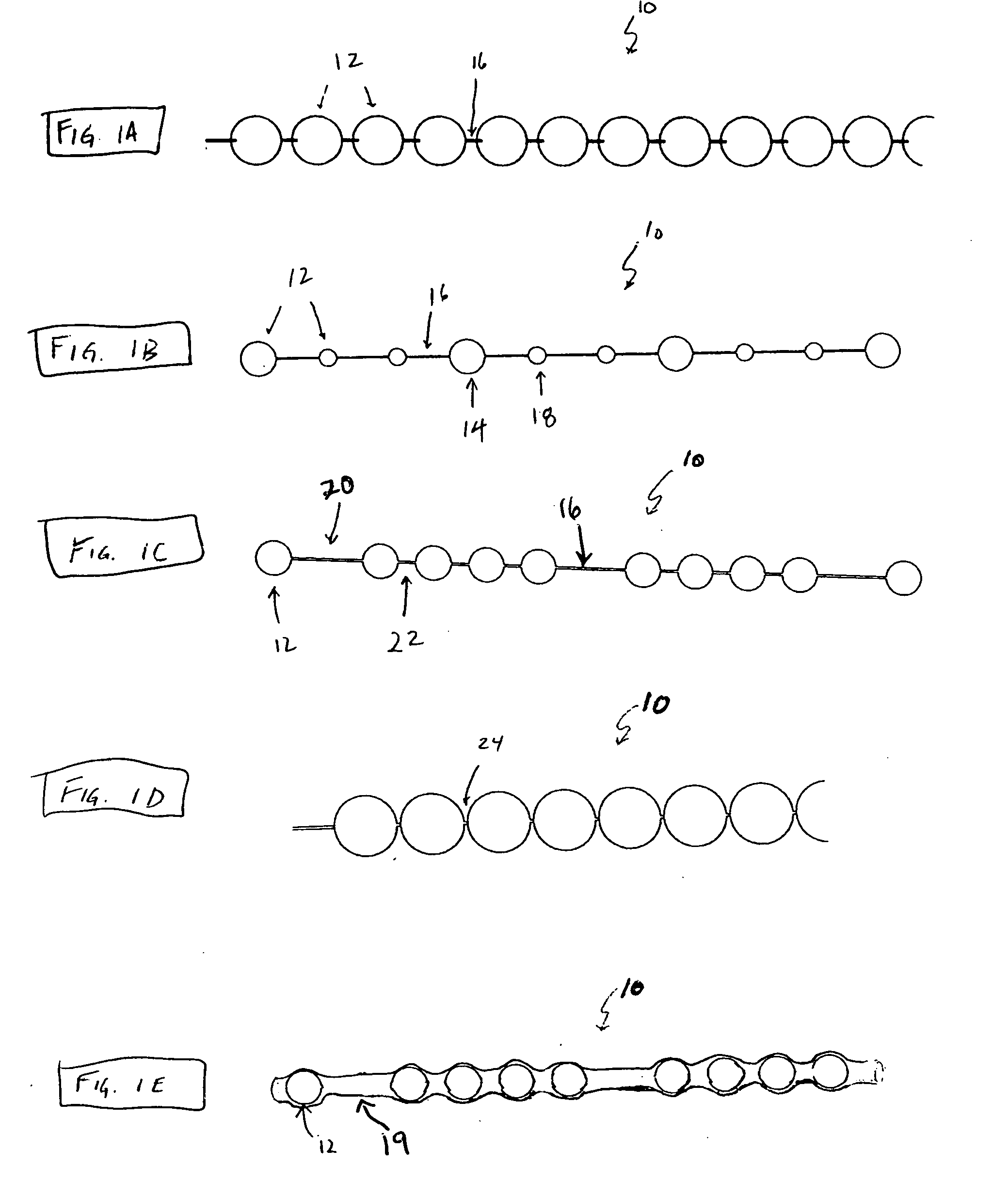
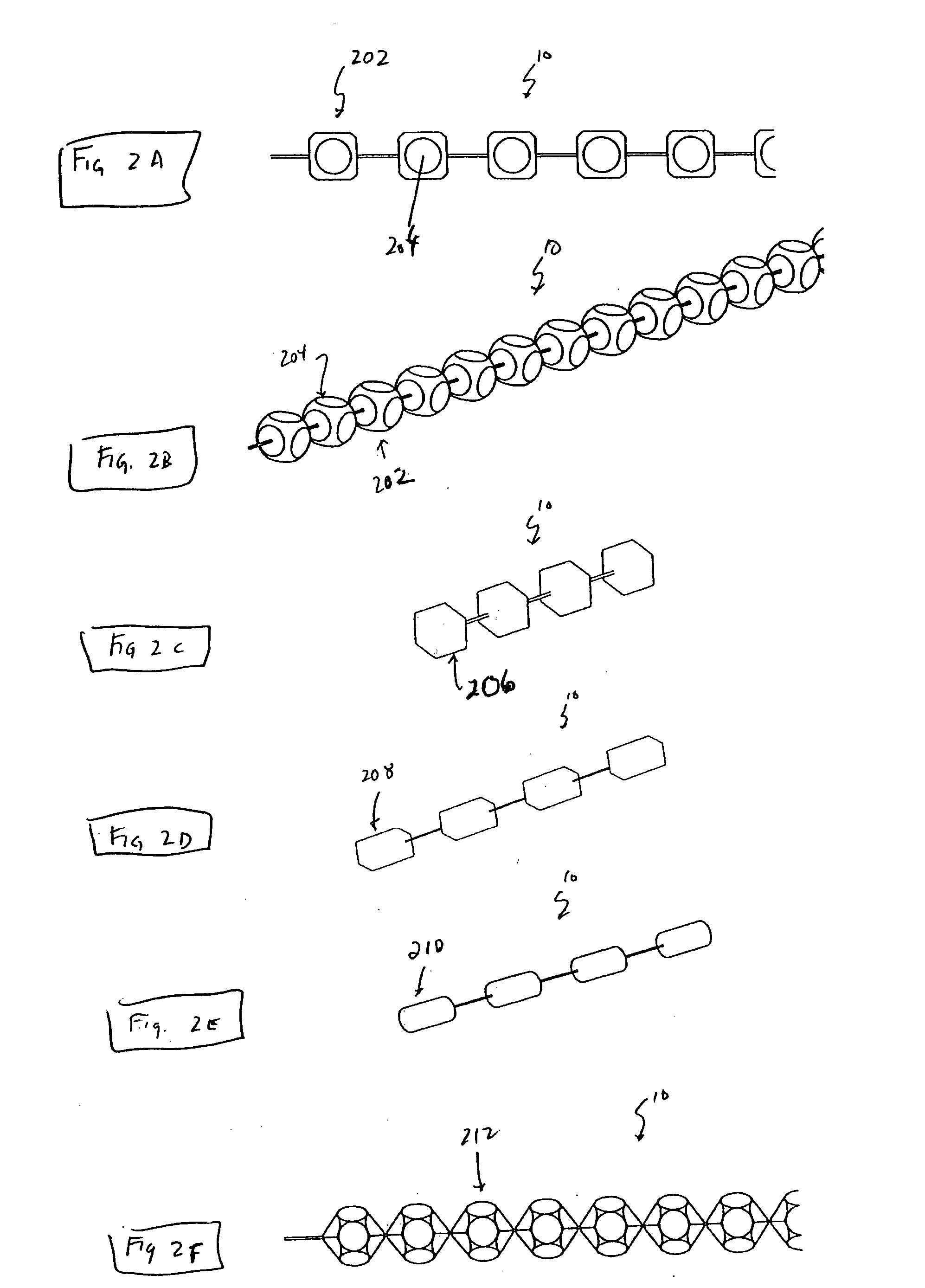
[0140]FIG. 8A to 8C shows an example of inserting an implant into a bone cavity 602. In this example, the bone cavity is part of a vertebral compression fracture. Other examples of bone disorders and fractures which may be distracted include, but are not limited to, tibial plateau fractures, femoral head necrosis, osteonecrosis of the hip, knee injury, etc. FIG. 8A shows an applicator 502 inserted into a vertebral compression fracture 804 through the vertebral pedicle 808; the applicator is inserting an implant 10 into the collapsed region. The implant is shown as a linear array of pellets 12. These segments of the implant may be continuously added to the bone cavity to first fill and pack ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com