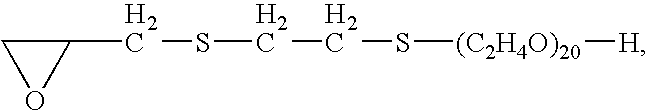Method for electrodeposition of bronzes
a technology of electrodeposition and bronze, which is applied in the direction of electrolytic coating, surface reaction electrolytic coating, coating, etc., can solve the problems of not being electrolytically deposited in acid, having economic and technical disadvantages, and having their limits in the deposition of tin-copper alloys with high copper content, etc., to achieve the effect of facilitating the adjustment of ph, facilitating the solubility of metal salts, and contributing significantly to the stability of baths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2b (
[0112] Example 2b (Yellow Bronze) [0113] 4 g / L Sn2+[0114] 18 g / L Cu2+[0115] 286 g / L methanesulfonic acid [0116] 32.2 g / L aromatic nonionic wetting agent [0117] 6 mg / Lbrightener [0118] 2 g / L oxidation inhibitor [0119] 50 mg / L stabilizer / complexing agent
[0120] Example 3 (White Bronze) [0121] 5 g / L Sn2+[0122] 10 g / L Cu2+[0123] 240 g / L methanesulfonic acid [0124] 32.2 g / L aromatic nonionic wetting agent [0125] 6 mg / Lbrightener [0126] 2 g / L oxidation inhibitor [0127] 25 mg / L stabilizer / complexing agent
example 4 (
[0128] Example 4 (Matte White Bronze) [0129] 18 g / L Sn2+[0130] 2 g / L Cu2+[0131] 258 g / L methanesulfonic acid [0132] 9 g / L aromatic nonionic wetting agent
[0133] To improve the hardness and / or ductility of the deposited bronze coatings the contents of zinc and / or bismuth indicated below as examples are added to the electrolyte. Additional data on the corresponding process conditions and other properties of the individual coatings can be seen in Table 1.
[0134] Example 5 (High Ductility) [0135] 4 g / L Sn2+[0136] 18 g / L Cu2+[0137] 238 g / L methanesulfonic acid [0138] 32.2 g / L aromatic nonionic wetting agent [0139] 3 mg / Lbrightener [0140] 2 g / L oxidation inhibitor [0141] 25 mg / L stabilizer / complexing agent [0142] 20 g / L ZnSO4
[0143] Example 6 (Hardness) [0144] 4 g / L Sn2+[0145] 18 g / L Cu2+[0146] 238 g / L methanesulfonic acid [0147] 32.2 g / L aromatic nonionic wetting agent [0148] 2 g / L oxidation inhibitor [0149] 25 mg / L stabilizer / complexing agent [0150] 0.1 g / L Bi3+
[0151] Example 7 (Yellow ...
example 8 (
[0159] Example 8 (Yellow Bronze) [0160] 2 g / L Sn2+[0161] 8 g / L Cu2+[0162] 400 g / L methanesulfonic acid [0163] 2.5 g / L aromatic nonionic wetting agent [0164] 1 g / L fatty alcohol ethoxylate [0165] 4 g / L oxidation inhibitor
[0166] Example 9 (White Bronze) [0167] 4 g / L Sn2+[0168] 8 g / L Cu2+[0169] 400 g / L methanesulfonic acid [0170] 1 g / L aromatic nonionic wetting agent [0171] 40 mg / L substituted dithioglycol [0172] 4 g / L oxidation inhibitor
[0173] With these exemplary electrolyte compositions coatings with specific properties were deposited under the process conditions listed in the following table.
Coating / AmountsExamplein wt %Properties of coatingNo.SnCuZnBiHardnessDuctilityGlossColor11090——180 HV50++YesRed 2a2080——283 Hv50±YesYellow 2b2080——317 HV50±YesYellow34060——360 HV50±YesWhite49010———−NoWhite52080——+++YesYellow62080—345 HV50−YesYellow72080——++YesYellow
[0174] When introducing elements of the present invention or the preferred embodiment(s) thereof, the articles “a,”“an,”“the,” ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com