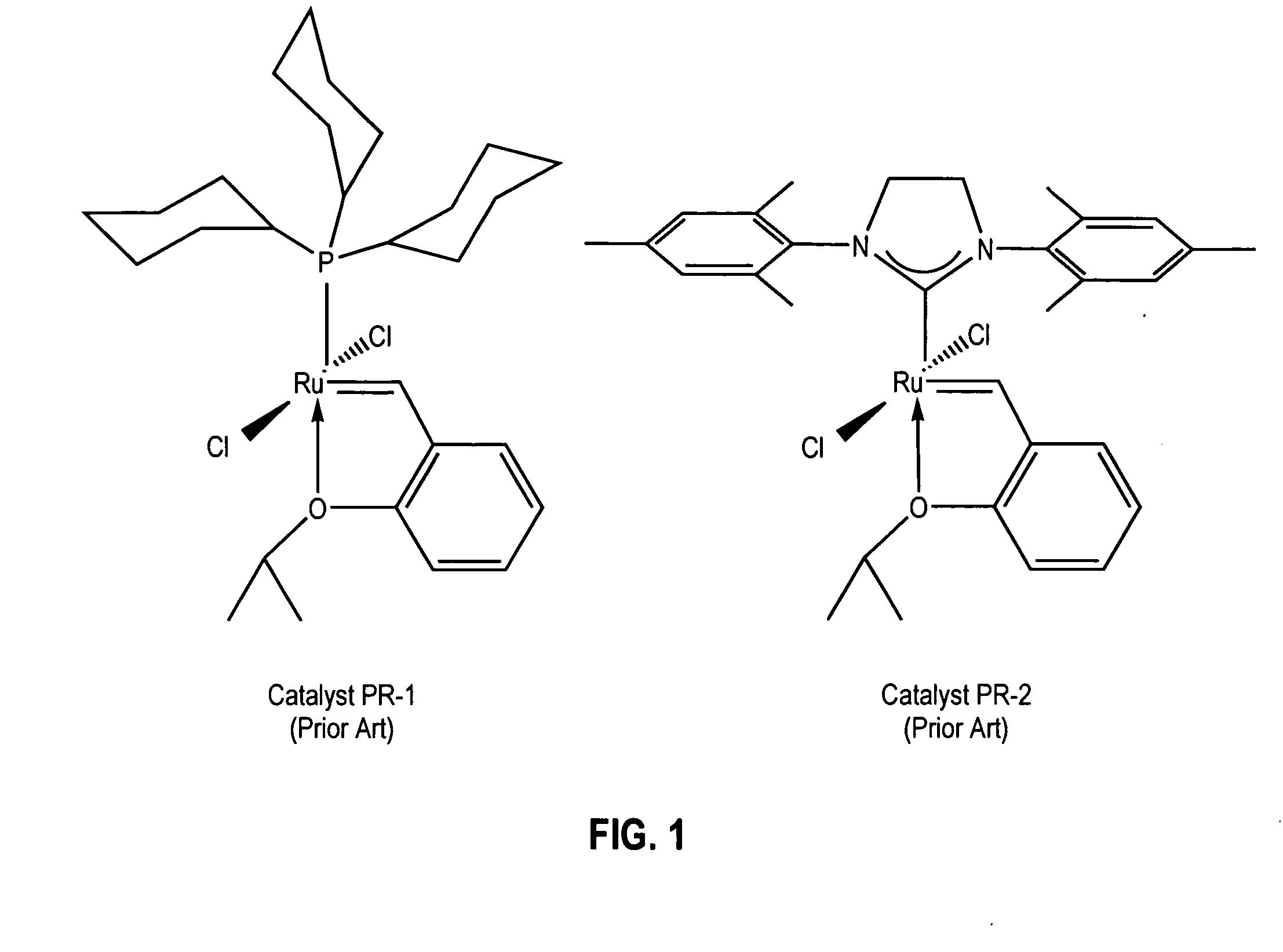
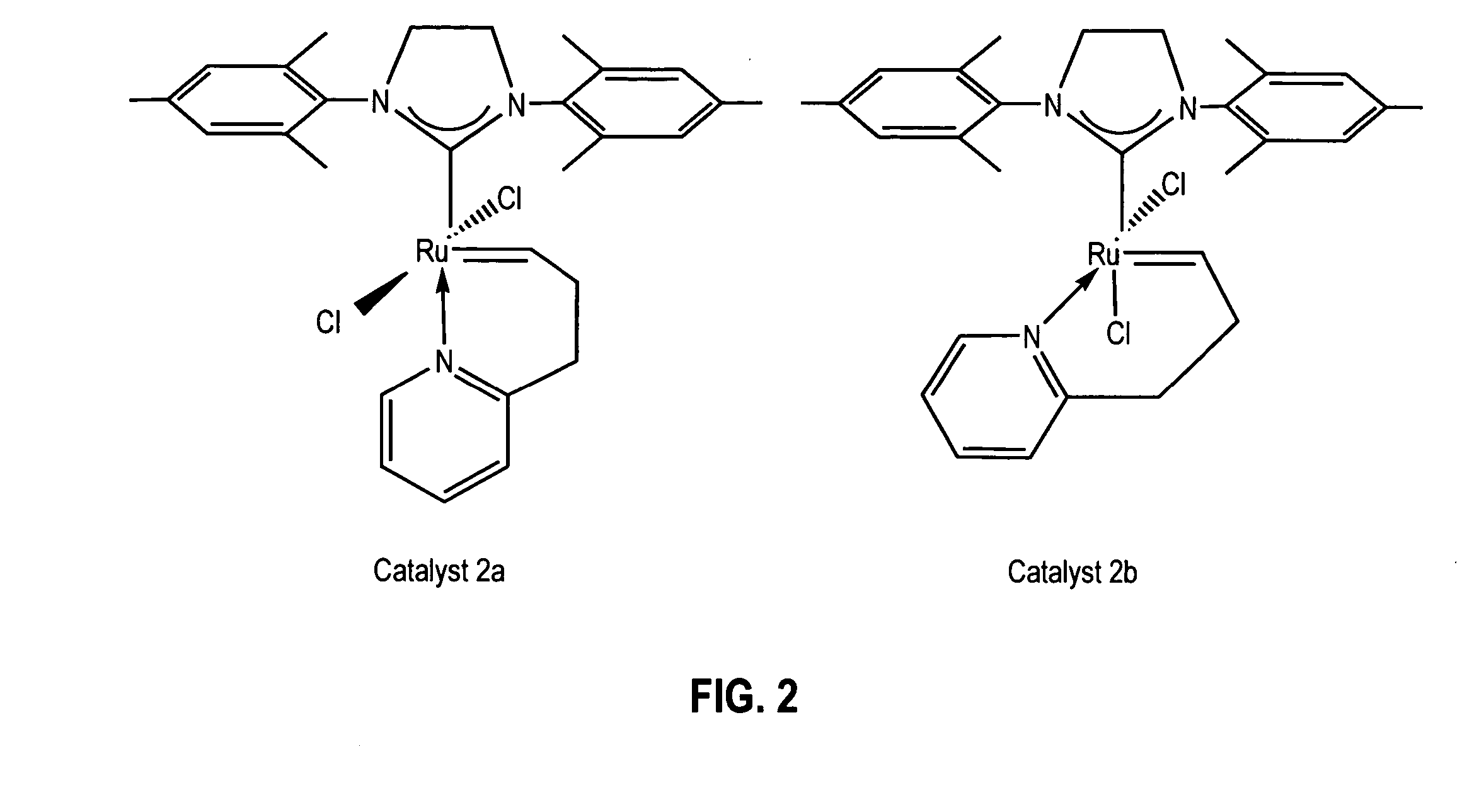
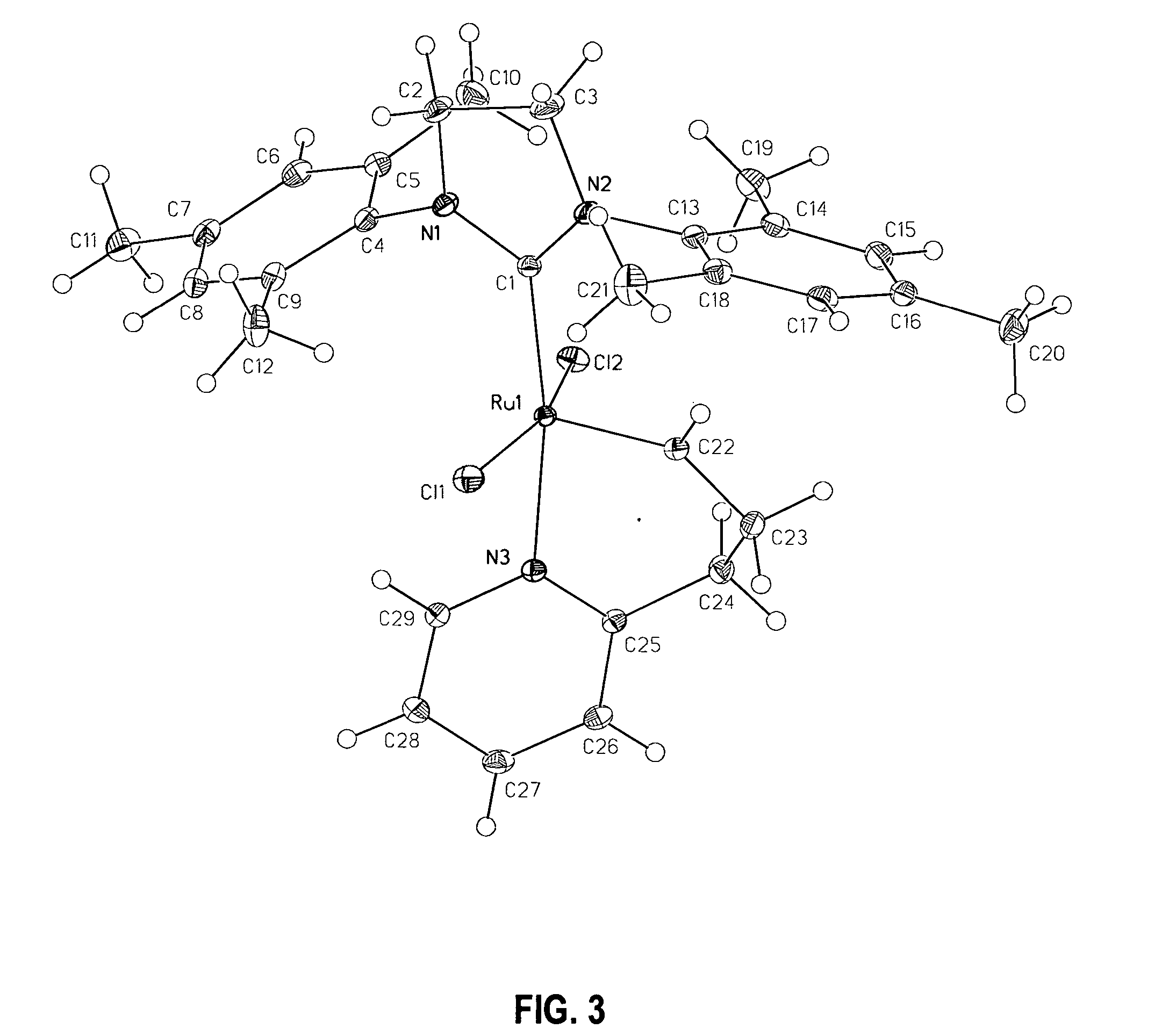
Latent, high-activity olefin metathesis catalysts containing an N-heterocyclic carbene ligand
a technology of olefin metathesis and ligand, which is applied in the field of olefin metathesis catalysts, can solve the problems of limited lifetime, modest degree of latency control, and lower activity of bisphosphine complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Catalyst 2a: Method A
[0111] A 250 mL round bottom Schlenk flask equipped with a stir bar was charged with complex 1, (sIMes)(PCy3)(Cl)2Ru═CHPh, (10.0 g; 11.8 mmol). The flask was capped, sparged with argon for 15 minutes, and charged with anhydrous CH2Cl2 (118 mL) via cannula. 2-(3-butenyl)pyridine (2.4 g, 17.7 mmol) was then added via syringe and the reaction mixture was heated to 40° C. for 5-6 hours. The reaction mixture was concentrated to dryness and the residue triturated with degassed, chilled methanol. The solid was collected on a frit and washed with chilled methanol (2×25 mL) to give catalyst 2a, (sIMes)(Cl)2Ru(CH(CH2)2—C,N-2-C5H4N)—Cs, (5.6 g; 9.4 mmol) as a pale green solid upon drying. Yield: 80%.
example 2
Synthesis of Catalyst 2a: Method B
[0112] In the glove box a vial was charged with 2-(3-butenyl)pyridine (24 mg, 0.18 mmol) and CH2Cl2 (2 mL). Complex 3, (sIMes)(py)2(Cl)2Ru═CHPh, (86 mg; 0.12 mmol) was then added as a solid and the reaction allowed to stir at room temperature for 30 minutes. The volatiles were removed under vacuum and the residue triturated with hexanes. The solid was collected, washed with hexanes (2×1 mL) and dried under vacuum to give catalyst 2a, (sIMes)(Cl)2Ru(CH(CH2)2—C,N-2-C5H4N)—C, (60 mg; 0.10 mmol) as a pale green solid upon drying. Yield: 85%. 1H NMR (CD2Cl2): δ 18.46 (t, 3JHH=2.7 Hz, 1 H, Ru═CH), 7.64 (d, 3JHH=4.8 Hz, 1 H, Py), 7.52 (t, 3JHH=7.2 Hz, 1 H, Py), 7.14 (d,3JHH=7.8 Hz, 1H, Py), 7.07 (s, 4 Mes), 6.99 (t, 3JHH=6.9 Hz, 1 H, Py), 4.09 (s, 4 H, sIMes), 3.55 (t, 3JHH=5.7 Hz, 2H, CH2-Py), 2.50 (s, 12 H, Mes-CH3), 2.41 (s, 6 H, Mes-CH3), 1.70 (m, 2 H, Ru═CH—CH2). 13C{1H} NMR (CD2Cl2): δ 339.18 (Ru═CHCH2), 216.52 (Ru—C(N)2), 162.64, 158.34, 149.54, 13...
example 3
Conversion of Catalyst 2a to Catalyst 2b
[0113] In the glove box, a 0.1 M solution of catalyst 2a in CD2Cl2 was prepared and transferred to an NMR tube, which was capped and taken out of the glove box. The NMR tube was left in an oil bath at 40° C. and the reaction was monitored by 1H NMR spectroscopy. The ratio of 2b to 2a in the mixture was 30 / 70 after 24 hours; 60 / 40 after 48 hours; 70 / 30 after 72 hours; and 78 / 22 after 96 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| structure | aaaaa | aaaaa |
| stability | aaaaa | aaaaa |
| structures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com