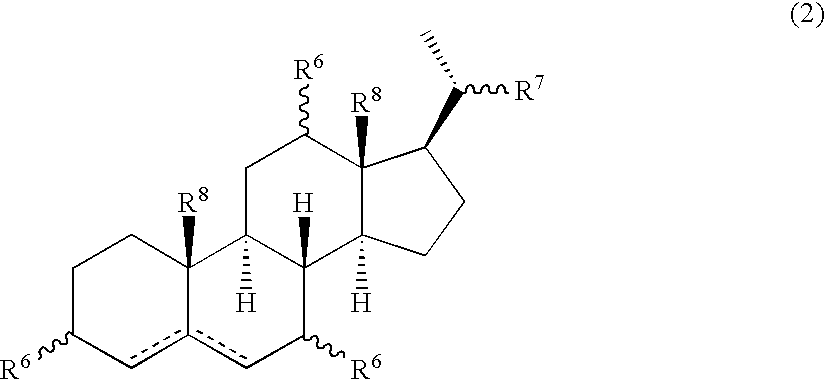
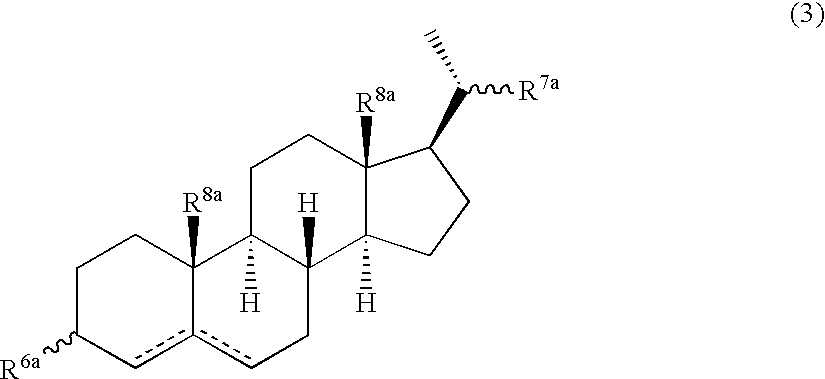
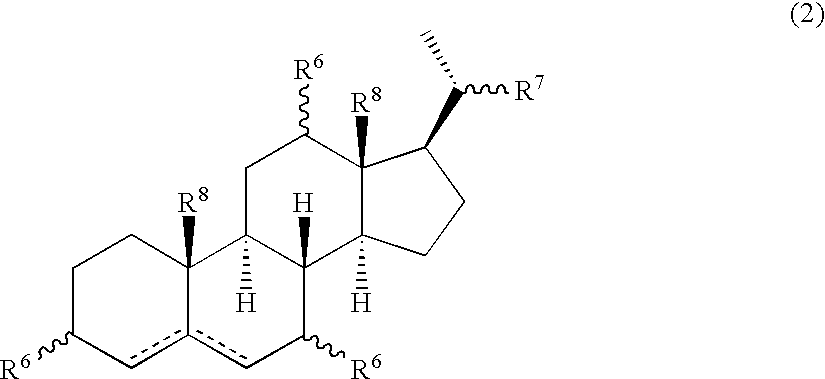
Multifunctional molecular complexes for gene transfer to cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N4-5′-Aminopentylspermidine Hydrochloride 8
N4-(4-cyanobutyl)-N1,N8-Bis(tert-butyloxycarbonyl)-S-permidine (6)
[0252] A solution of N1,N8-Bis(tert-butyloxycarbonyl) spermidine (2.92 g, 8.45 mmol, 1.0 eq) [S.Nagarajan and B. Ganem, J. Org. Chem., 50, 5735-37 (1985)] in acetonitrile (125 mL) was treated with N,N-diisopropylethylamine (3.534 mL, 20.0 mmol, 2.4 eq), potassium iodide (2.81 g, 16.90 mmol, 2.0 eq), and 5-chlorovaleronitrile (1.902 mL, 16.90 mmol, 2.0 eq). The resulting homogeneous solution was heated to reflux for 2 hours. The mixture was treated with additional N,N-diisopropylethylamine (1.767 mL; 1.2 eq), potassium iodide (1.41 g, 1.0 eq) and 5-chlorovaleronitrile (0.951 mL, 1.0 eq), and refluxed an additional 18 hours. Thin layer chromatography (TLC) indicated no remaining starting material. The acetonitrile was removed under vacuum, and the residue taken up in chloroform (250 mL). This solution was washed with water (200 mL), dried (Na2SO4), and stripped...
example 2
Preparation of N4-(5-(P-3′-propionyl galactosyl-β1″-4′-thioglucoside) aminopentyl)speridine 9
S-(Succinimidyl-β-3′-propionyl)hepta-O-acetyl galactosyl-β1′4-thioglucoside (10)
[0255] A solution of S-β-3-propionyl hepta-O-acetyl galactosyl-β1-4-thioglucoside [M. Elofsson, S. Roy, B. Walse and J. Kihlberg, Carb. Res., 246, 89-103 (1993)] (4.60 g, 6.35 mmol) in 1:1 isopropanol / chloroform (100 mL) was treated with N-hydroxysuccinimide (0.73 g, 6.35 mmol) and N,N′-dicyclohexylcarbodiimide (1.31 g, 6.35 mmol). After stirring at room temperature for 19 hours, the mixture was cooled to 40 for 1 h and filtered. The solvent was removed from the filtrate under vacuum to give a white solid that was recrystallized from 2-propanol (3.43 g). The isolated product was 92% pure by high performance liquid chromatography (HPLC). 1H NMR (CDCL3): δ 2.0-2.16 (7s, 21.0H), 2.85 (s, 3.8H), 2.85-3.1 (m, 4.2H), 3.65 (m, 0.7H), 3.78 (t, 1.0H), 3.88 (t, 1.0H), 4.11 (m, 4.0H), 4.54 (m, 2.8H), 4.97 (m, 1.8H), 5.11 ...
example 3
Preparation of N4-(5-[N2,N6-bis(P-3′-propionyl galactosyl-p 1-4-thioglucoside)lysyl-N6(β-3′-propionyl galalactosyl-β1-4-thioglucoside)lysyl]-amino)pentylspermidine Acetate Salt 18
[0259] For comparative purposes, the CAS style name of the compound 18 set out above is as follows: 4-[(N[2[N2,N6-bis(3-[4-O(β-D-galactopyranosyl)-β-D-glucopyranosylthio]propionyl)lysyl]N6-(3-[4-O-(β-D-galactopyranosyl)-β-D-glucopyranosylthio]-propionyl)lysinamido)pentyl]-1,8-diamino-4-azaoctane, Acetate Salt.
N4-(5-[N2,N6-bis(β-3′-propionyl hepta-O-acetyl galactosyl-β1-4-thioglucoside)lysyl N6-(β-3′-propionyl hepta-O-acetyl galactosyl-β1-4-thioglucoside) lysine (14)
[0260] To a solution of lysyl-lysine (0.25 g, 0.64 mmol) in 1:1 water / acetonitrile (100 mLs) was added N,N-diisopropylethylamine (0.336 mls, 1.95 mmol, 3.0 eq) and compound 10 (1.859, 2.25 mmol) and stirred at room temperature for a few minutes until homogeneity was achieved. The pH was closely monitored at regular intervals, and N,N-diisoprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com