Modified-release tablet of bupropion hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0098] 1. Modified Release Tablet Formulations
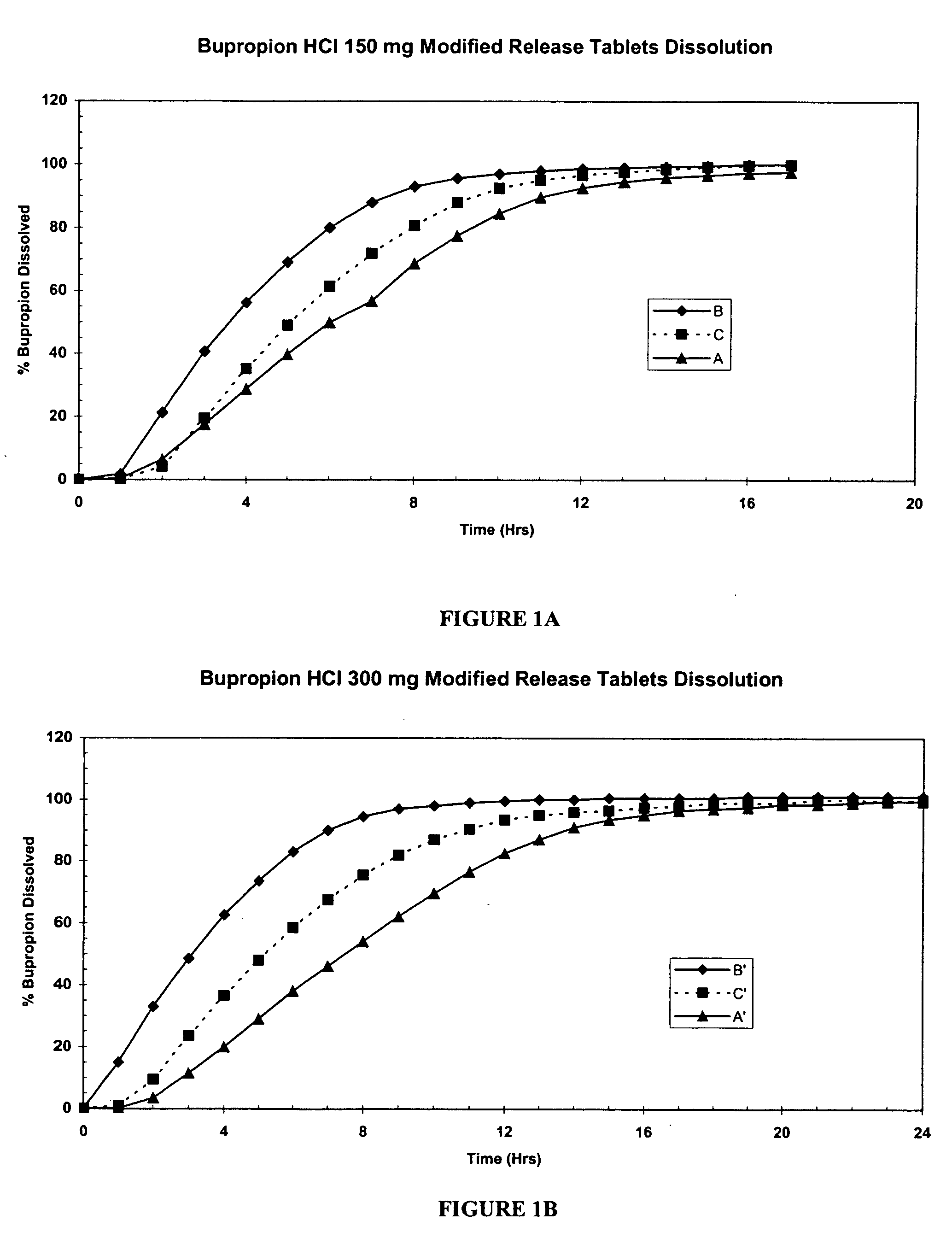
[0099] Three different core formulations were prepared for each of the 150 mg and 300 mg modified release bupropion hydrochloride tablets as shown in Table 1:
TABLE 1 CORE FORMULATION150 mg300 mgABCA′B′C′Ingredients(mg / %)1(mg / %)(mg / %)(mg / %)(mg / %)(mg / %)Bupropion 150 / 81.1 150 / 82.4150 / 79 300 / 79 300 / 87.6 300 / 83.5hydrochlorideBinder2 5.3 / 2.865.3 / 2.95.3 / 2.810.6 / 2.8 10.6 / 3.1 10.6 / 2.95Lubricant3 4.7 / 2.54 4.7 / 2.58 4.7 / 2.46 9.4 / 2.48 9.4 / 2.74 9.4 / 2.61Purified water4******Total dry weight 160 / 86.48 160 / 87.91 160 / 83.77 320 / 84.43 320 / 93.47 320 / 89.02of core
1The mg / % values represent the proportion of the ingredient in relation to the tablet dry weight
3Glyceryl behenate (Compritol 888 ATO)
4Evaporated during drying
[0100] The water is first heated to 60±5° C. The binder (polyvinyl alcohol) is next dissolved in the water to homogeneity and then passed through a 0.7 mm mesh screen and allowed to cool to a temperature of no mor...
example 2
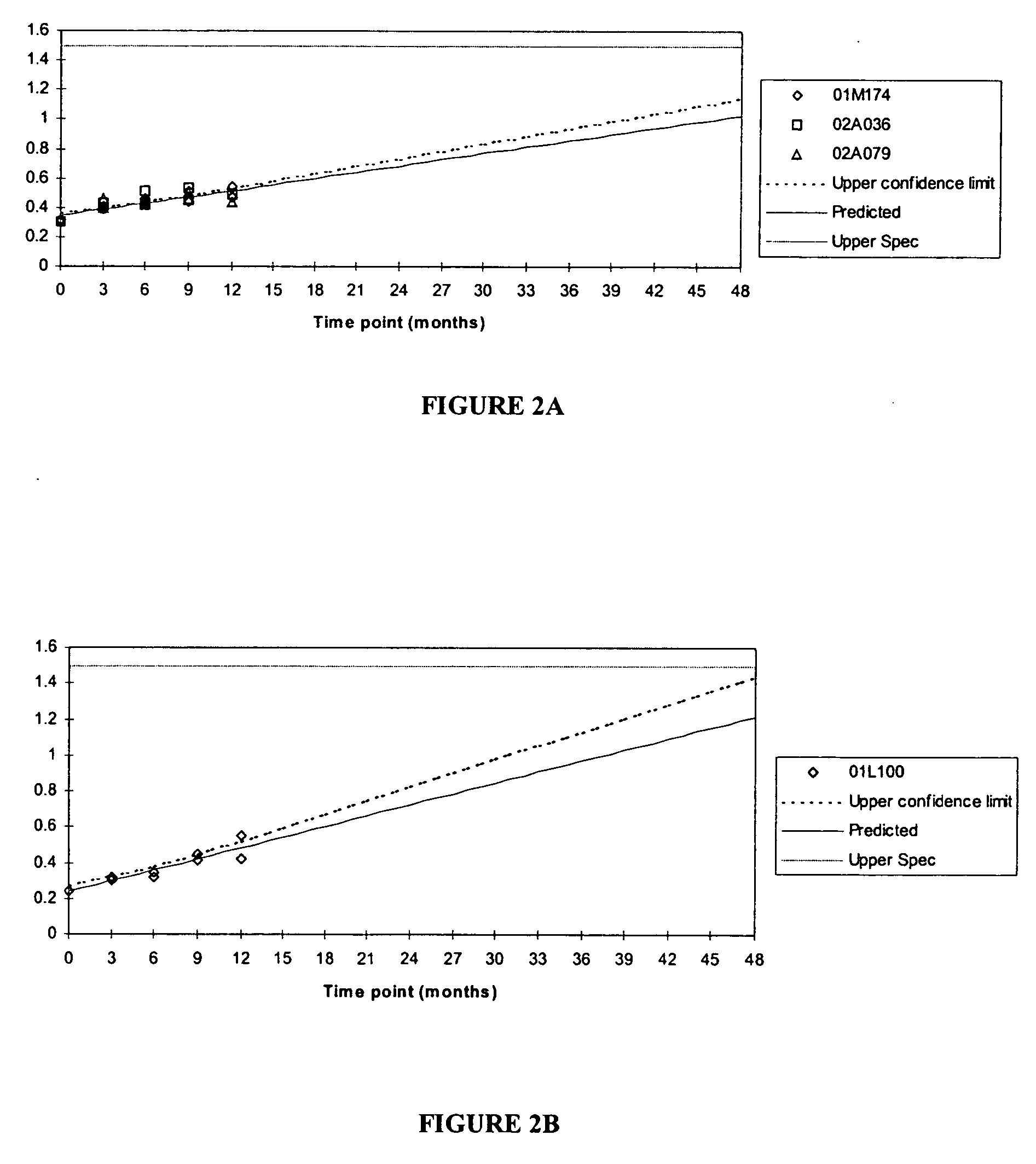
[0118] 1. The Moisture Barrier is not an Enteric Coat
[0119] The purpose of this study was to show that the modified release bupropion hydrochloride tablets of the invention are not enteric coated. The modified release formulation is based on a tablet core comprising bupropion hydrochloride, a binder and a lubricant. The tablet core is coated with a control-releasing coat, which functions to control the release of the bupropion hydrochloride. The control-releasing coated tablet cores are subsequently coated with a moisture barrier, which substantially impedse or retards absorption of moisture.
[0120] The release of the drug was measured spectrophotometrically by a two-stage dissolution procedure using USP enteric coating dissolution conditions method B (Basket at 75 rpm) to evaluate the tablet integrity. The results of the tests are shown in Tables 11 and 12:
TABLE 11 Acid Stage: % dissolvedTimeof 300 mg modified release bupropion HCl tablets(hr)V1V2V3V4V5V6MeanSD00.00.00.00.00.00....
example 3
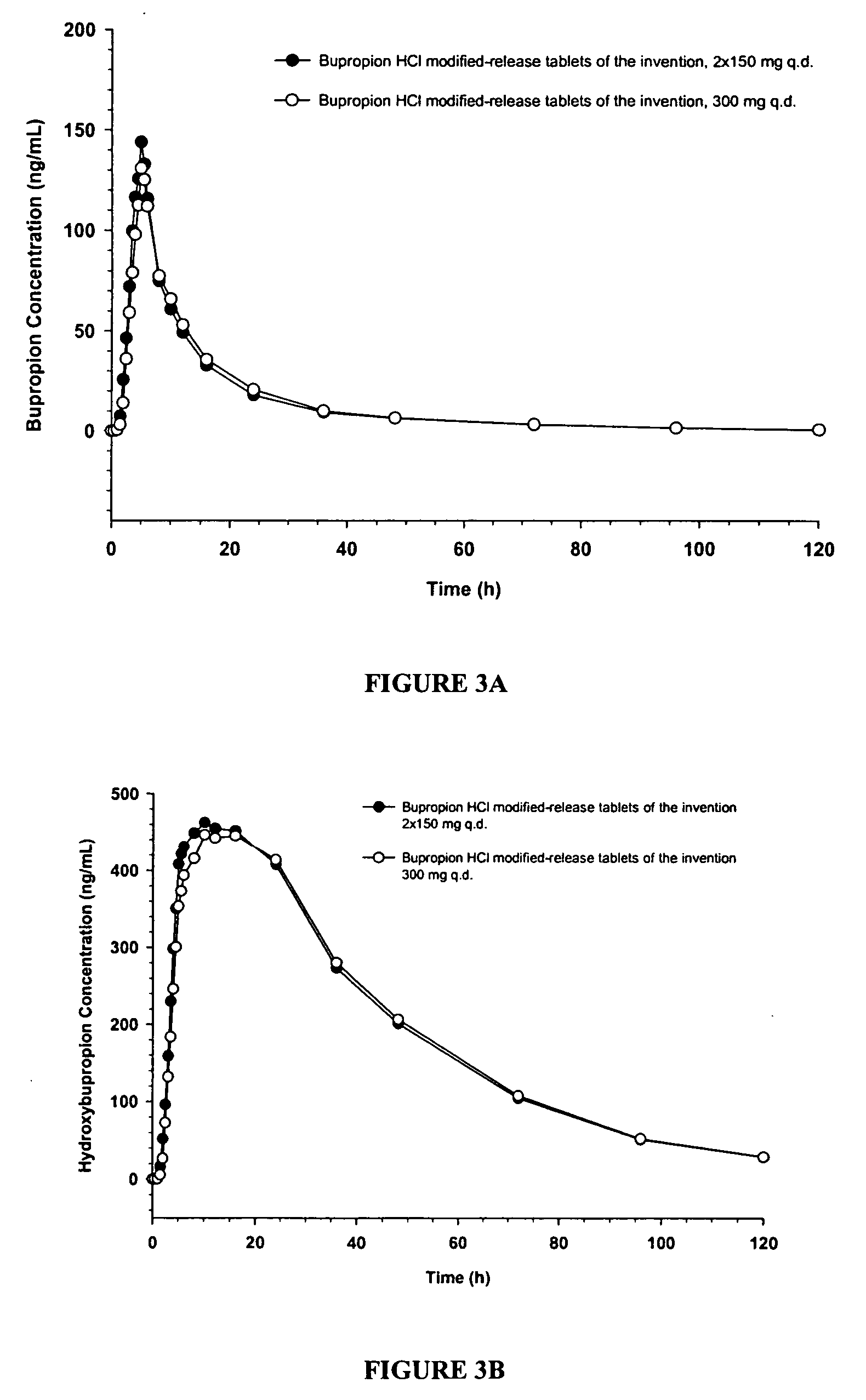
[0130] The objective of this study was to investigate the dosage strength equivalency of the following test 150 mg and 300 mg product strengths of Bupropion HCl modified-release tablets under fasting conditions. A two-way, crossover, open-label, single-dose, fasting, dosage strength equivalency study of two strengths (150 mg and 300 mg) of bupropion HCl modified-release tablets of the invention was conducted. The modified-release tablets of the invention were administered once daily in normal healthy non-smoking male and female subjects.
[0131] The study design involved a 2-period, 2-treatment, single-dose crossover design under fasting conditions. The study periods were separated by a 3-week washout period. A total of 36 subjects (19 Male, 17 Female) enrolled for the study of which 35 of the subjects (19 Male, 16 Female) completed the study. Subjects were administered the following treatments: [0132] A) 2×150 mg q.d. modified-release bupropion hydrochloride tablets of the invention...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com