Aerosol deliver apparatus IV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
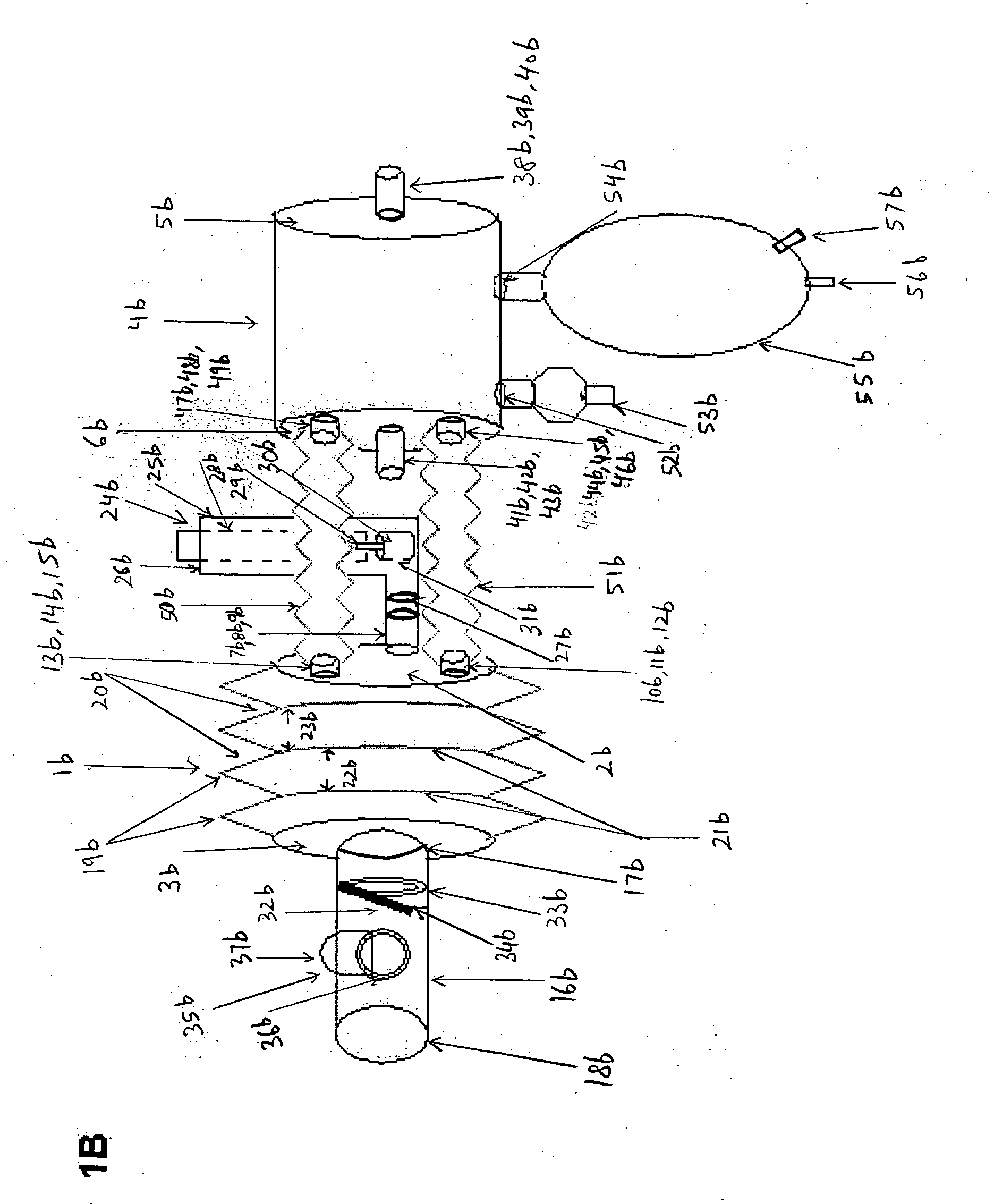
[0074] The present invention will now be described in detail by reference to the drawing figures, where as like parts as indicated by like reference numerals.
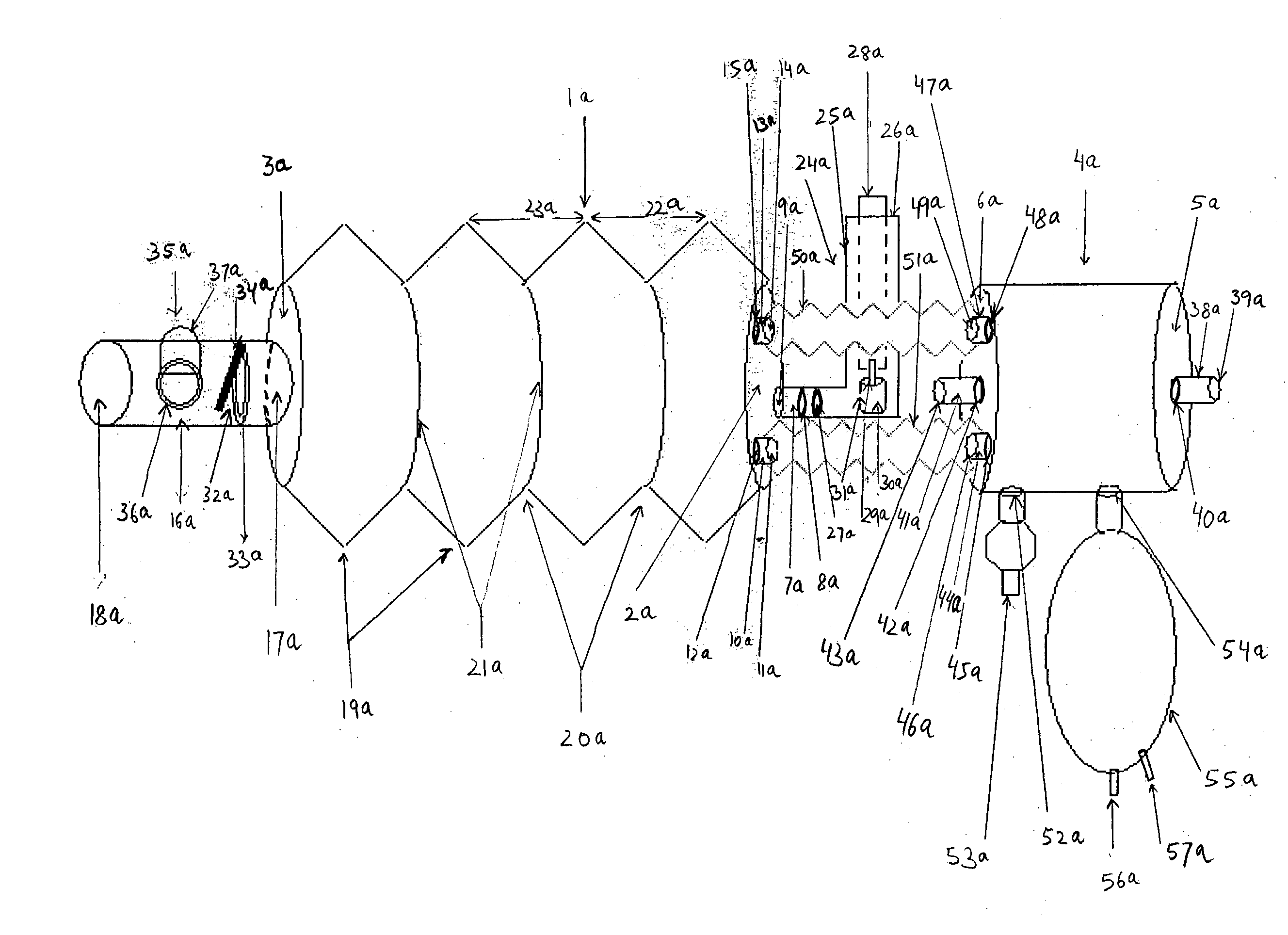
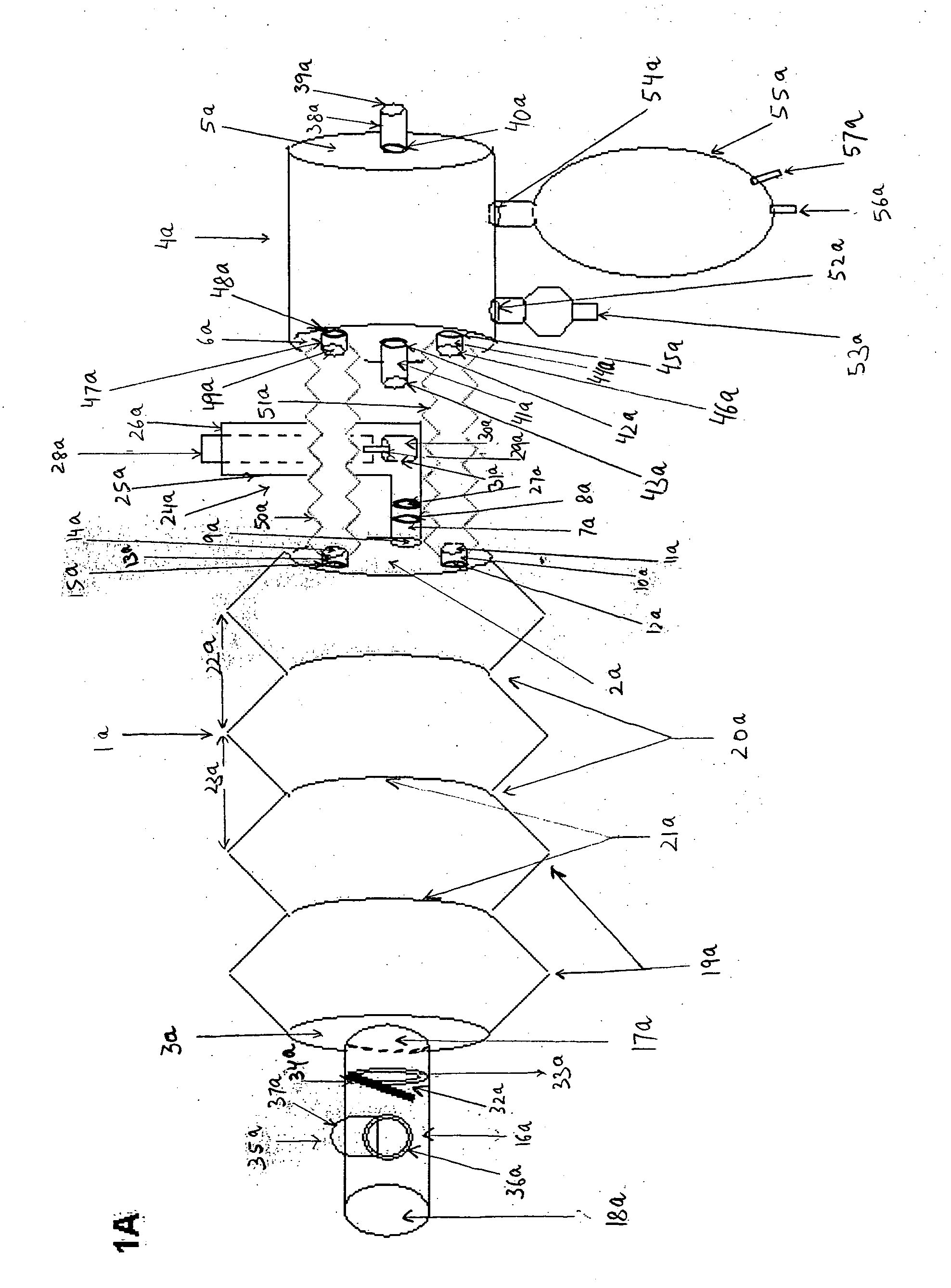
[0075]FIG. 1A is a plan view of the longitudinal length of aerosol delivery apparatus IV according to one embodiment of the present invention, incorporating the features described in the summary of the invention. FIG. 1A is a plan view of the invention that may be used with a metered dose inhaler (MDI) or a nebulizer. The illustration here describes the use of this device preferentially with an MDI. The device has two hollow chambers, a metered dose inhaler chamber 1a, and a nebulizer chamber 4a. The MDI chamber 1a has an inlet end 2a and an outlet end 3a. The nebulizer chamber 4a similarly has an inlet end 5a and an outlet end 6a. The inlet end 2a has three hollow cylindrical inlet tubes, a central tube 7a and two peripheral tubes 10a and 13a located at three o'clock to nine o'clock positions, respectively. The central hollow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com