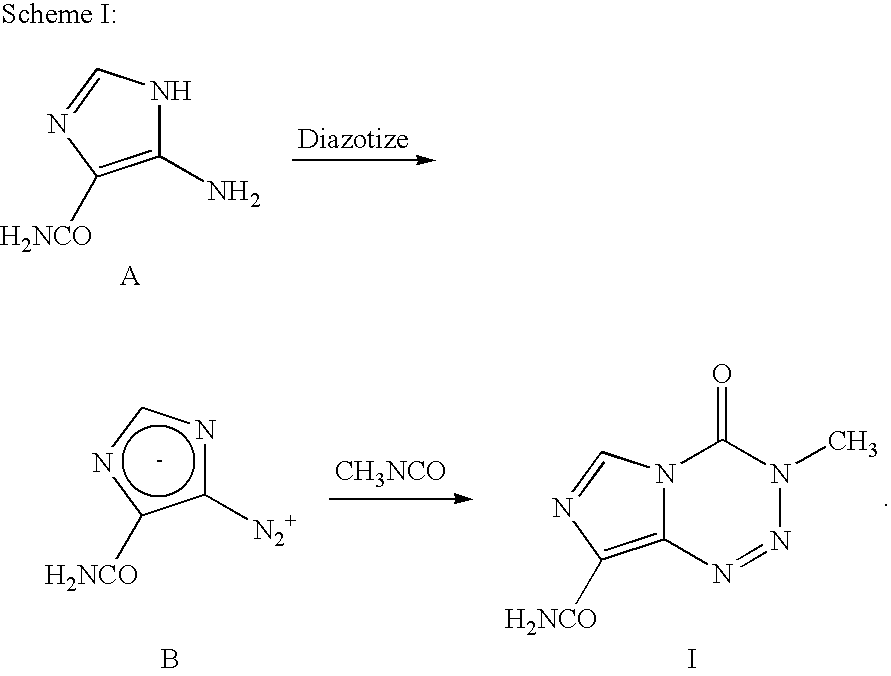
Synthesis of temozolomide and analogs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
3-Methyl-8-aminocarbonvl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (Temozolomide)
[0059] Step A: Preparation of 2-cvano-N-(1,1-dimethylethyl)-2-[(diphenylmethylene)amino]-acetamide
[0060] The imine 3 (700 g, 3.178 mol) and CH2Cl2 (7 L) were placed into a 22 L three-necked flask equipped with a nitrogen inlet, a gas outlet tube, reflux condenser, thermometer, mechanical stirrer, and maintained under a positive pressure of nitrogen. 1,1-Dimethylethyl-isocyanate (442 mL, 3.870 mol) was added to this stirred mixture at 0° C., and after stirring for 10 min a solution of potassium t-butoxide in THF (1.0 M in THF, 3.88 L, 3.88 mol) (as supplied by Aldrich) was added slowly (1 hour). The solution was stirred at 0° C. for 4 hours, when the reaction mixture had become a very thick paste with a deep brown color, and thin layer chromatography (EtOAc / hexanes=¼) indicated that no more starting material was present. The resulting mixture was quenched with saturated NH4Cl solution (5 L), and the o...
example 2
Preparation of Intermediates and Reagents
[0073] Part A: 2-Cyano-N(1,1-dimethylethyl)-2-(hydroxyimino)acetamide 13
[0074] Amide 11 (3.11 g, 22.18 mmol) (Bhawal, B. M.; Khanapure, S. P.; Biehl, E. R.; Syn. Commun., 1990, 20, 3235) dissolved in CH2Cl2 (100 mL) was placed into a 500 mL 3-necked round-bottom flask equipped with a stirring bar. The solution was cooled to 0° C. (ice bath) and NOCl (Fluka) was bubbled through until the reaction mixture turned a brick-red color. The reaction mixture was stirred at 0° C. for 30 min and then at room temperature for 18 hours. The precipitate was collected and washed with CH2Cl2 (25 mL) to afford the product as a white solid (2.88 g, 17.0 mmol).
[0075]1H NMR (400 MHz, DMSO-d6, δ): 7.70 (s, 1H), 3.32 (s, 1H), 1.32 (s, 9H); mp: 218-219° C.
Part B: 2-Amino-2-cyano-N-(1,1-dimethylethyl)acetamide 13
[0076] Oxime 13 (2.5 g, 14.78 mmol), Al amalgam (0.81 g) and distilled H2O (100 mL) were placed into a 250 mL round-bottom flask equipped with a stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com