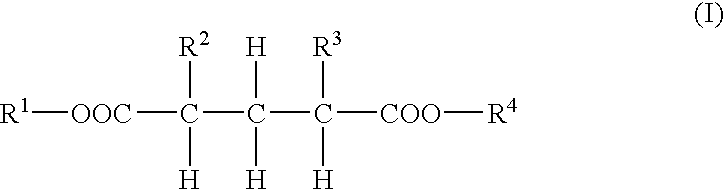
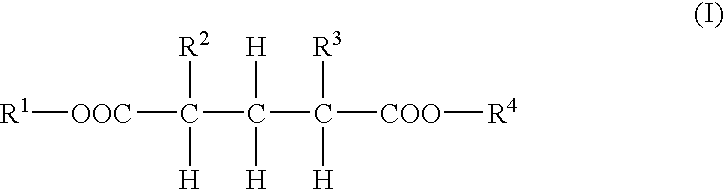
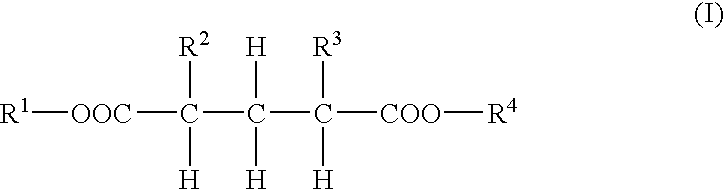
Dibasic acid diesters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
[0032] 2,4-Diethyl-1,5-pentanediol (160.3 g) (trade name: Kyowadiol PD-9, Kyowa Yuka Co., Ltd., purity: 93.9%), 156.6 g of potassium hydroxide (purity: 86%) and 102.2 g of carbon number 12 paraffin mixture (trade name: Kyowasol C1200-H, Kyowa Yuka Co., Ltd.) were placed in a 1L nickel autoclave equipped with a reflux condenser, a pressure control valve and an electric furnace capable of temperature control, and heated with stirring under 1 MPa. The generated hydrogen gas was measured with a gas meter, and the progress of reaction was monitored. The generation of the gas was confirmed at around 230° C., and the reaction was continued at a temperature maintained in the range of 250 to 270° C. After the temperature reached 250° C., 89.4 l of hydrogen was generated in 3.5 hours. The reaction was continued for further 30 minutes, during which 0.8 l of hydrogen gas was generated. The amount of the generated hydrogen agreed with the theoretical value and the rate of reaction was 100%. Afte...
example 1
Synthesis of bis(2-ethylhexyl) 2,4-diethylglutarate (Compound 1)
[0033] In a reaction flask were placed 2,4-diethylglutaric acid (92.50 g), 2-ethylhexanol (66.00 g) and toluene (157.3 g), and the mixture was stirred well and p-toluenesulfonic acid monohydrate (2.67 g) was added thereto, followed by reflux for 5 hours. After cooling to room temperature, the reaction mixture was neutralized with a 0.1 wt % aqueous solution of sodium hydroxide and then washed with water. The solvent was distilled away from the reaction mixture at 135° C. in vacuo, whereby 146 g of Compound 1 (yield: 98.4%) was obtained. The physical properties of Compound 1 were as follows. [0034]1H-NMR (CDCl3, δ ppm): 3.98 (m, 4H), 2.32 (m, 2H), 1.94 (m, 1H), 1.75 (m, 1H), 1.59 (m, 4H), 1.28 (m, 18H), 0.95 (m, 18H) [0035] IR (cm−1): 2972, 2942, 2887 (C—H), 1745 (C═O), 1465, 1388, 1261, 1163 (C—O) [0036] MS (m / z): 414 (M+) [0037] Density (kg / m3): 929 (25° C.) [0038] Kinematic viscosity (cSt): 9.47 (40° C.), 2.53 (100° ...
example 2
Synthesis of bis[5,7,7-trimethyl-2-(1,3,3-trimethylbutyl)octyl]2,4-diethylglutarate (Compound 2)
[0039] In a reaction flask were placed 2,4-diethylglutaric acid (92.06 g), 5,7,7-trimethyl-2-(1,3,3-trimethylbutyl)octyl alcohol (28.32 g) and toluene (30.23 g), and the mixture was stirred well and p-toluenesulfonic acid monohydrate (1.13 g) was added thereto, followed by reflux for 4 hours. After cooling to room temperature, the reaction mixture was neutralized with a 0.2 wt % aqueous solution of sodium hydroxide and then washed with water. 5,7,7-Trimethyl-2-(1,3,3-trimethylbutyl)octyl alcohol was removed from the reaction mixture by extraction with methanol (three times), whereby 82.9 g of Compound 2 (yield: 79.0%) was obtained. The physical properties of Compound 2 were as follows. [0040]1H-NMR (CDCl3, δ ppm): 4.01 (m, 4H), 2.32 (m, 2H), 1.96-1.04 (m, 28H), 0.88 (m, 54H) [0041] IR (cm−1): 2962, 2916, 2887 (C—H), 1743 (C═O), 1471, 1384, 1233, 1197, 1164 (C—O) [0042] Density (kg / m3): 8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com