High purity nanoscale metal oxide powders and methods to produce such powders
a technology of nanoscale metal oxide and fine powder, which is applied in the direction of lanthanide oxide/hydroxide, chemical/physical/physical-chemical processes, energy-based chemical/physical/physical-chemical processes, etc., can solve the problems of failure or defect of electronic and other applications, low volume, and high cost of methods, and achieve high purity and high volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
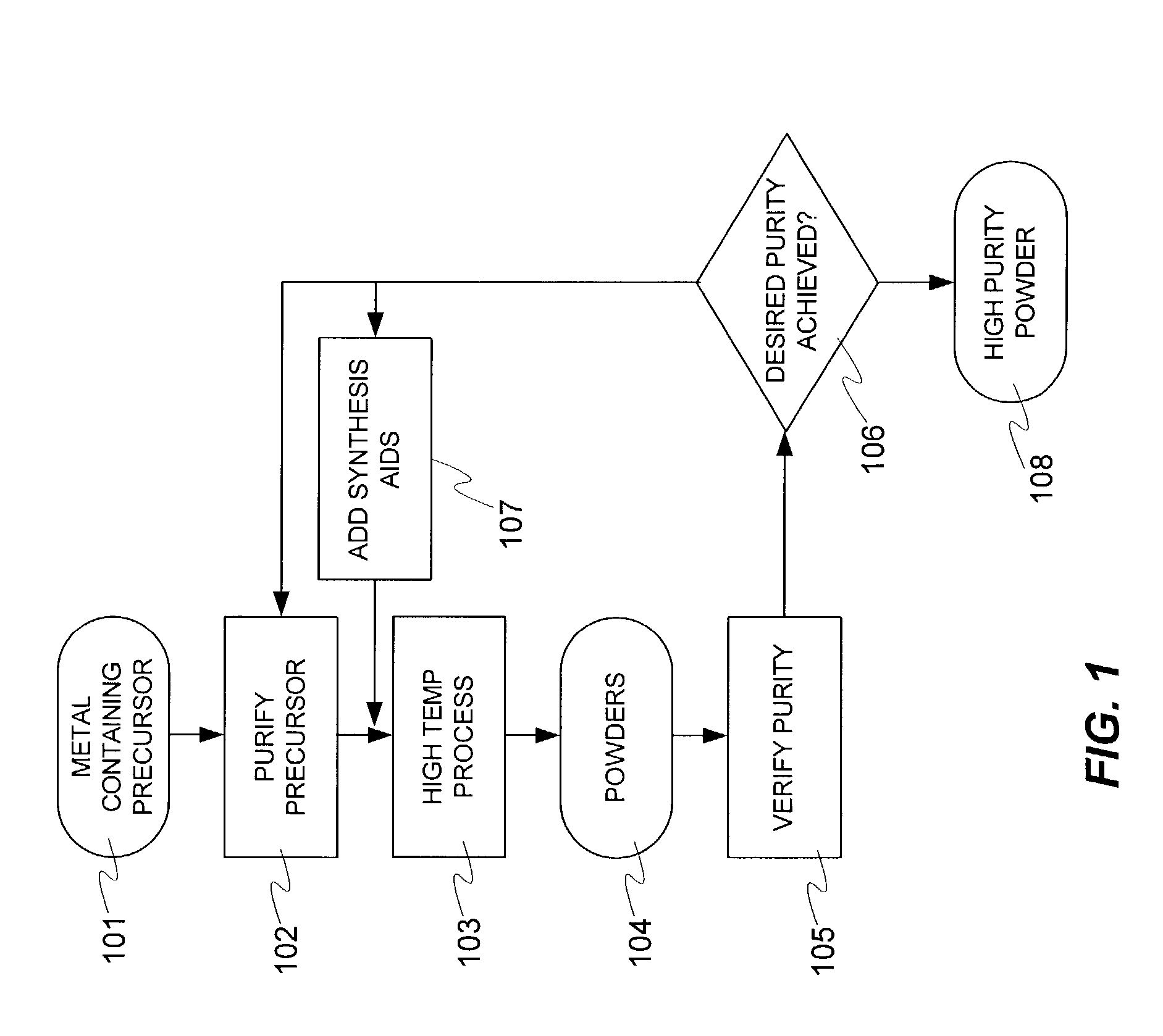
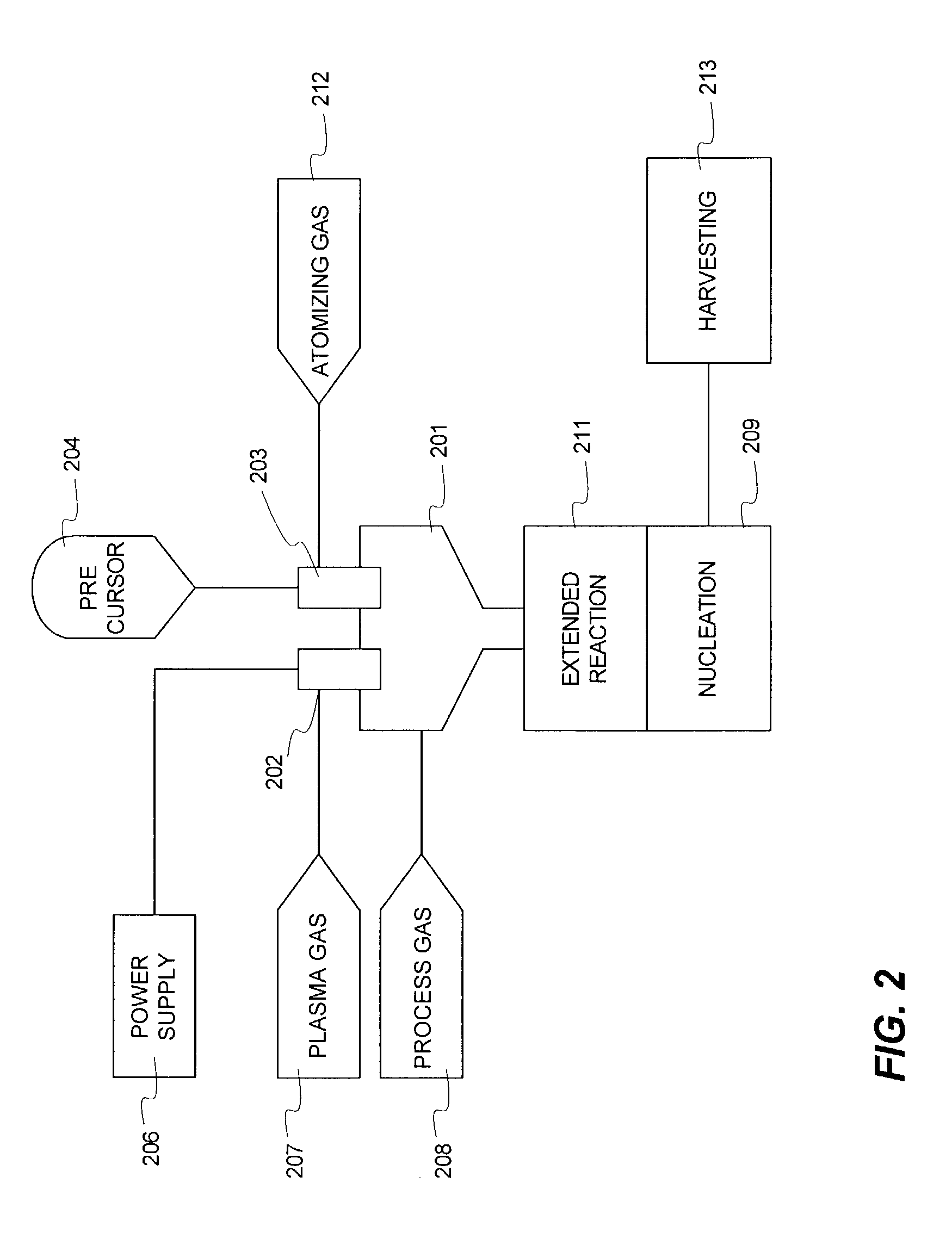
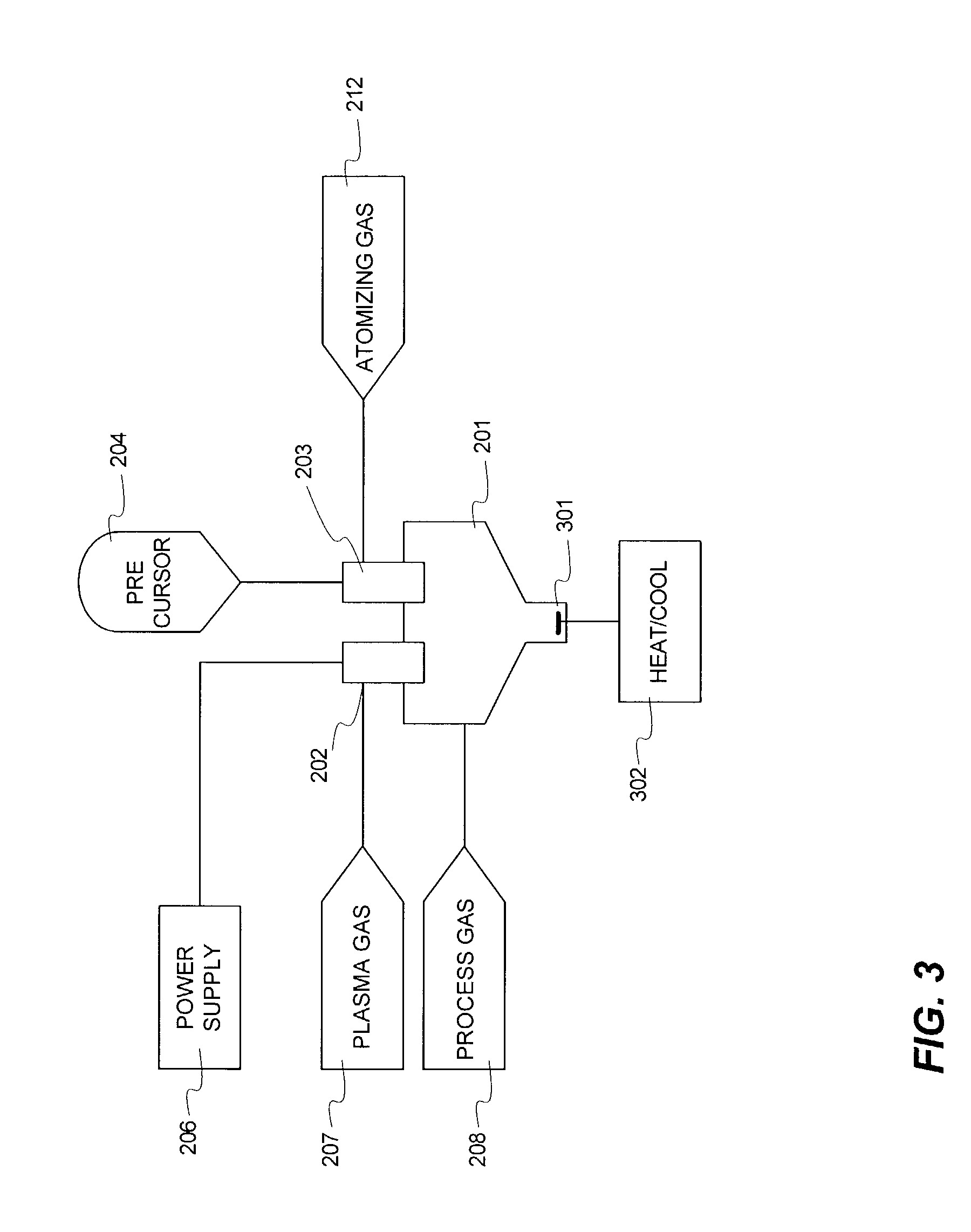
Magnesium acetate was dissolved in high purity water and pumped as a liquid into a plasma reactor. To ensure complete oxidation, pure oxygen was fed into the process. The core temperature of the plasma was greater than 6000° C., while the outer edge temperature was estimated to be greater than 3000° C. The plasma was produced using a DC arc and argon as the plasma gas. The precursor completely vaporized when it interacted with the plasma. The metal vapor oxidized completely. The vapor was slightly cooled to encourage the formation of nanopowder. The nanopowder containing stream was quenched in a converging diverging nozzle (to >103 ° C. / sec) in flowing oxygen. The powder was harvested using membrane bags and a venturi cyclone fed with compressed air for suction effect. The collected powder was high purity magnesium oxide (MgO) with surface area greater than 100 m2 / gm and mean size less than 10 nm. Over a two hour run, over 100 grams of powder were harvested. This ex...
example 2
In another run, magnesium acetate (Reagent Grade 1271R, Shepherd Chemical Company, Cincinnati, Ohio, USA) was dissolved in high purity water and pumped as a liquid into a plasma reactor. The feed had the following impurities on metal basis (K: 35 ppm, Na: 203 ppm, Fe: 88 ppm, Ca: 27 ppm, Ba: <9 ppm, Mn: 53 ppm, Sr: <9 ppm). To ensure complete oxidation, pure oxygen was fed into the process at a faster rate than in Example 1. The core temperature of the plasma was greater than 6000° C., while the outer edge temperature was estimated to be greater than 3000° C. The plasma was produced using a DC arc and argon as the plasma gas. The precursor completely vaporized when it interacted with the plasma. The vapor oxidized completely. The vapor was slightly cooled to encourage the formation of nanopowder. The nanopowder containing stream was quenched in a converging diverging nozzle (to >103 ° C. / sec) in flowing oxygen. The powder was harvested using membrane bags ...
example 3
Indium octoate and tin octoate were mixed in a specified ratio by metal basis. Indium-tin-oxide (ITO) powders with grain size less than 20 nm were produced using the process of Example 1. This example illustrated that fine powders, and more specifically nanopowders, of high purity complex multimetal oxides can be manufactured from fluids. Such high purity multimetal oxides are desired in numerous applications such as, but not limited to, coatings for EMI shielding, electronic, electromagnetic, device, thermal, catalytic, photonic, optical, electrochemical, chemical, sensor, other films / coatings, instrumentation, sputtering and biomedical applications.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com