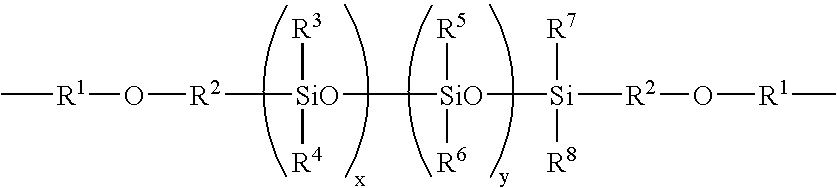
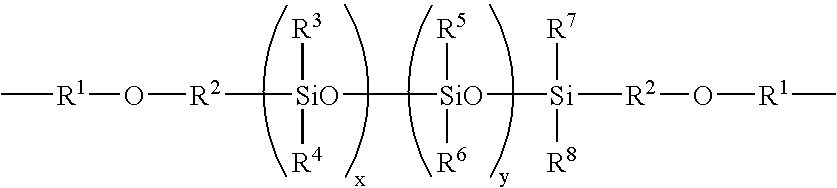
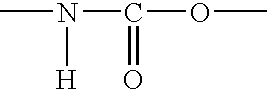Ocular lens material and process for producing the same
a technology of ocular lens and lipid, applied in the field can solve the problems of poor wettability of ocular lens material, etc., to improve wettability, improve wettability, and improve photo-irradiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 12 and 13
[0188] C.I. Reactive Blue 4 available from Mitsui BASF Dye Co., Ltd. was dissolved in 20 ml of distilled water in an amount of 0.0511 g to obtain a solution. To 20 ml of 2-propanol was added 1 ml of the above solution at 25.degree. C. A siloxane-containing polymer obtained in the same manner as in Example 2 (Example 12), and a siloxane-containing polymer obtained in the same manner as in Example 10 without ultraviolet-ray irradiation before saponification (Example 13) were immersed in the mixture for 16 hours, respectively. Next, these polymers were immersed in the same methanol aqueous solution containing sodium hydroxide as used in Example 2 for 6 hours at 25.degree. C., and then each polymer was taken out therefrom, transferred to distilled water, and taken out therefrom after 120 minutes.
[0189] All polymers assumed uniform and transparent blue color, and was equal to polymers obtained in Examples 2 or 10 in high oxygen permeability, and excellent lipid-stain resistance, surface ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com