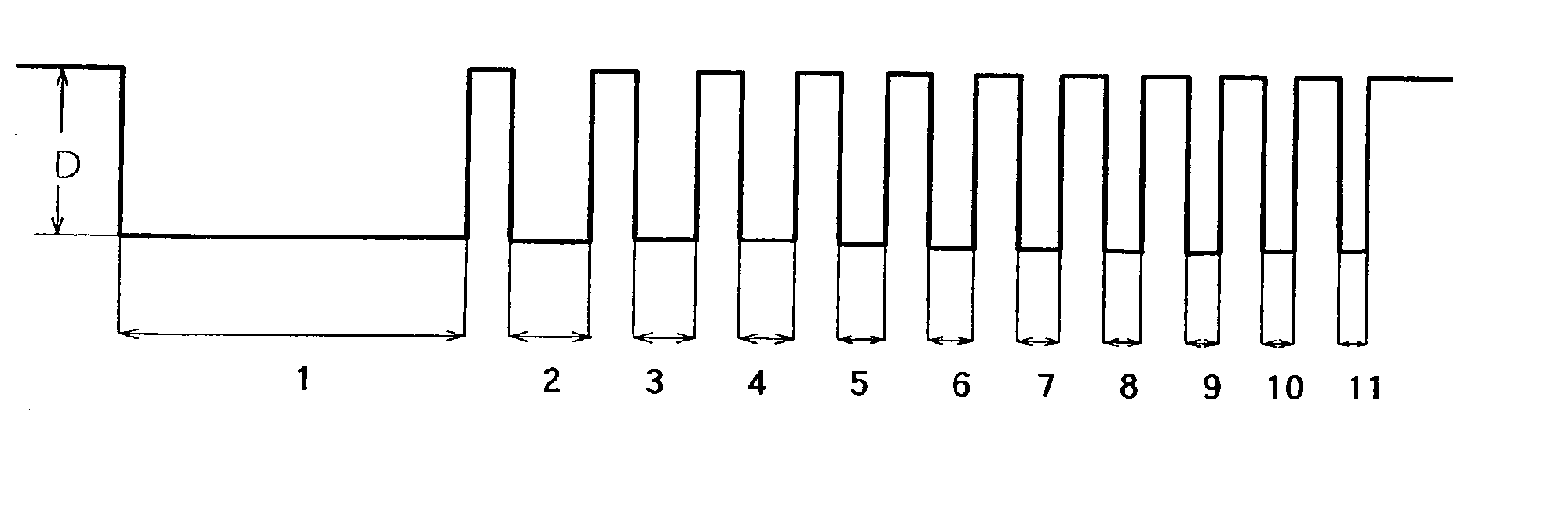
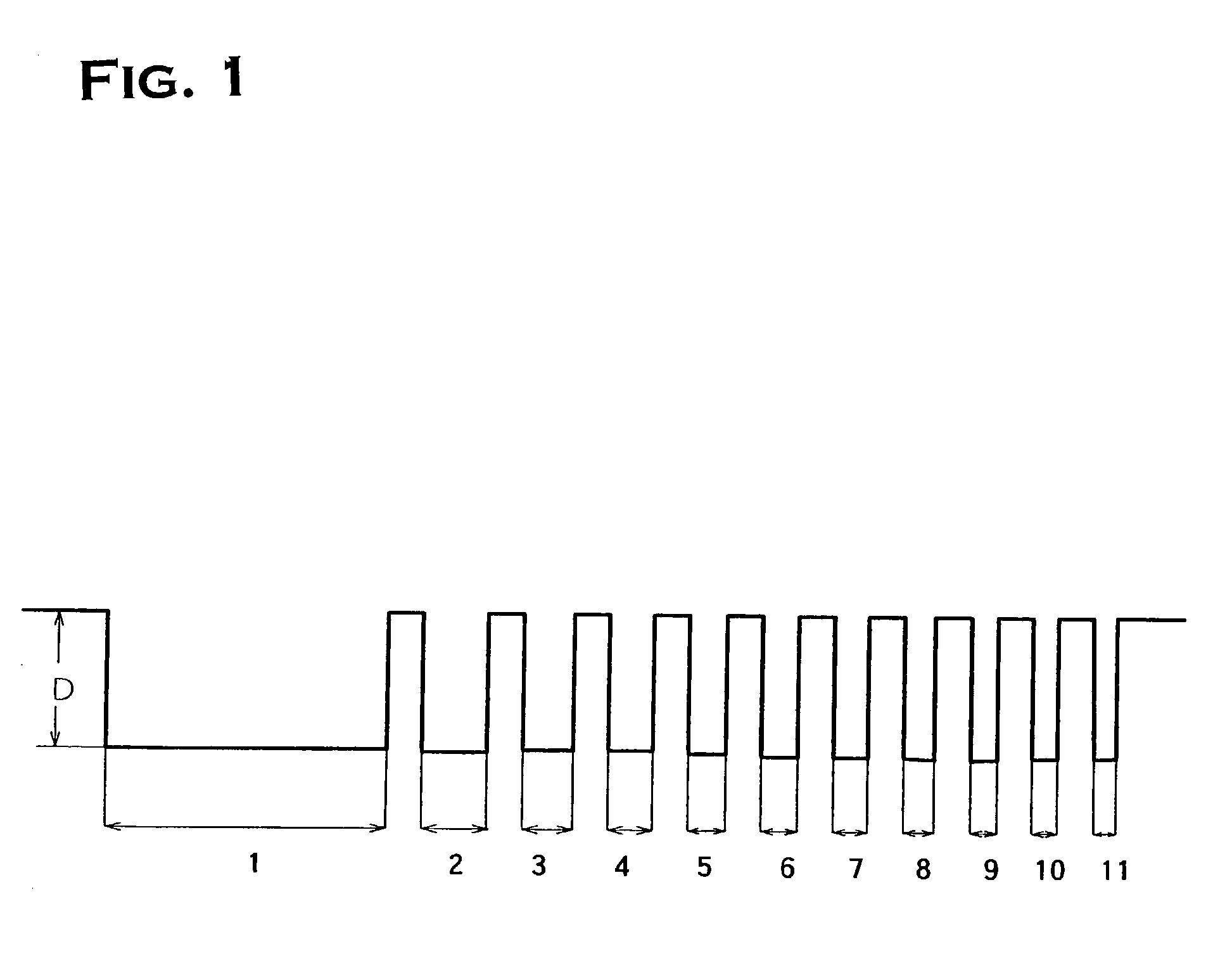
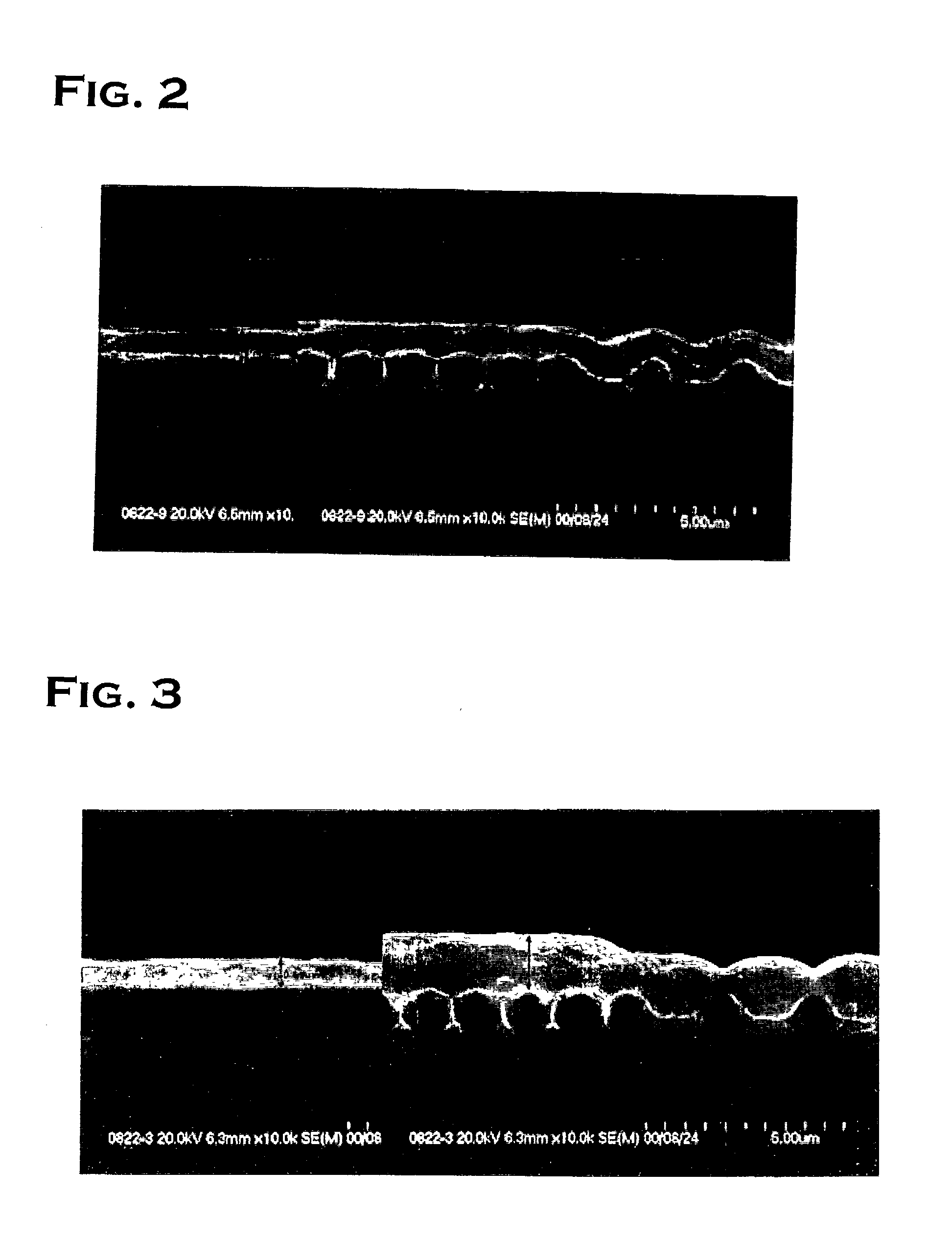
Copper plating solution for embedding fine wiring, and copper plating method using the same
a technology of fine wiring and copper plating solution, which is applied in the direction of basic electric elements, electrical equipment, semiconductor devices, etc., can solve the problems that the copper plating solution may not be uniformly plated with the copper sulfate solution, and the copper plating solution is not suitable for filling the fine gaps between the wiring patterns. , defects, voids and seams
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
1.0 20 0.01 2.5 0.5 0.55 .largecircle. .largecircle. Example 2 1.0 20 0.1 2.5 0.5 0.05 .largecircle. .largecircle. Example 3 1.0 20 1.0 2.5 0.5 0.07 .largecircle. .largecircle. Example 4 1.0 20 10.0 2.5 0.5 0.05 .DELTA. .DELTA. Example 5 1.0 20 20 2.5 0.5 0.06 X X Example 6 1.0 20 0.1 0.025 0.5 0.05 .largecircle. .DELTA. Example 7 1.0 20 0.1 2.5 0.5 0.06 .largecircle. .largecircle. Example 8 1.0 20 0.1 5.0 0.5 0.06 .largecircle. .largecircle. Example 9 1.0 20 0.1 25 0.5 0.05 .DELTA. .DELTA. Example 10 1.0 20 0.1 30 0.5 0.05 X X Example 11 1.0 20 10.0 2.5 0.5 0.05 .largecircle. .largecircle. Comparative 1.0 20 -- -- 0.5 0.74 X X Example 1 Comparative 1.0 20 -- 2.5 0.5 0.79 .largecircle. .largecircle. Example 2 Comparative 1.0 20 0.1 -- 0.5 0.06 .DELTA. X Example 3
[0043]
2TABLE 2 A Concentration [g / L] of a macromolecular, triol type surfactant of polypropylene glycol (PPG) having an average molecular weight of 5,000 B Concentration [mg / L] of 3,3-dithiobis-propane-sodium sulfonate C Con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com