Method of producing non-bovine chymosin and use hereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0070] Construction of a vector for the expression of camel chymosin
[0071] Unless indicated otherwise, recombinant DNA techniques were according to Sambrook et al., 1989.
[0072] 1.1 Cloning of Camelus dromedarius chymosin gene
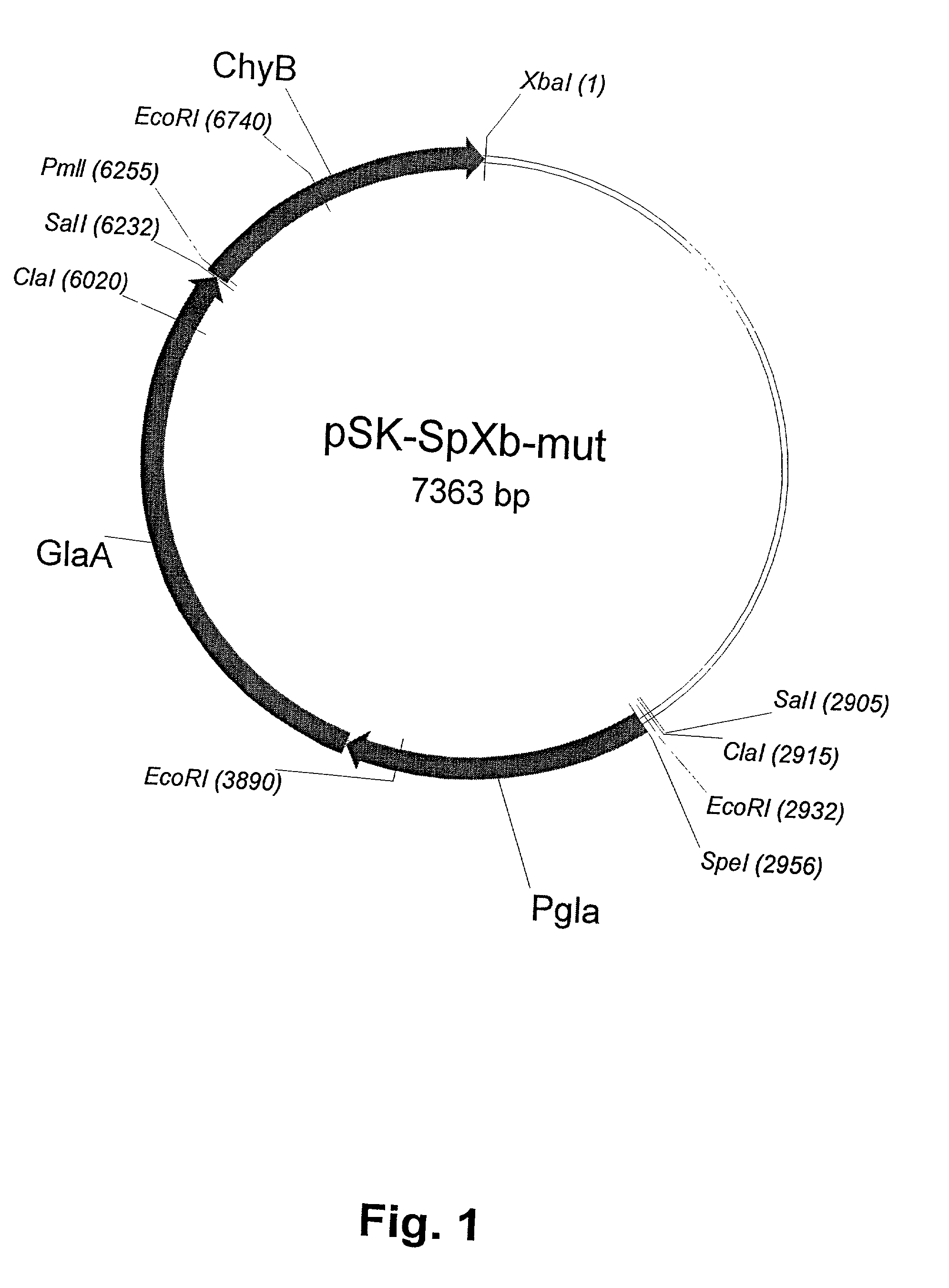
[0073] A DNA sequence containing a camel prochymosin (cd-prochymosin) coding sequence and adjacent 5' and 3' sequences of the pGAMpR vector (Ward et al., 1990) was amplified by PCR. The pGAMpR vector comprises, as a selection marker, the pyr4 gene of Neurospora crassa, which is capable of complementing a pyrG mutation in a recipient strain. mRNA was isolated from mucosal tissue of a 3 year old camel using a direct mRNA Kit (Quiagen, D-40724 Hilden, Germany). Based on this isolated mRNA, a cDNA template for PCR was generated by reverse transcription. For PCR amplification the following pair of primers were used:
1 cd-prochymosin forward: Pmll 5'-cacgtggcggAGTGGGATCACCAGG-ATCCCTCTG-3' (SEQ ID NO:1) cd-prochymosin reverse: Xbal 5'-tctagaggaTCAGATGGCCTTGGCCAGCCCCACG-...
example 2
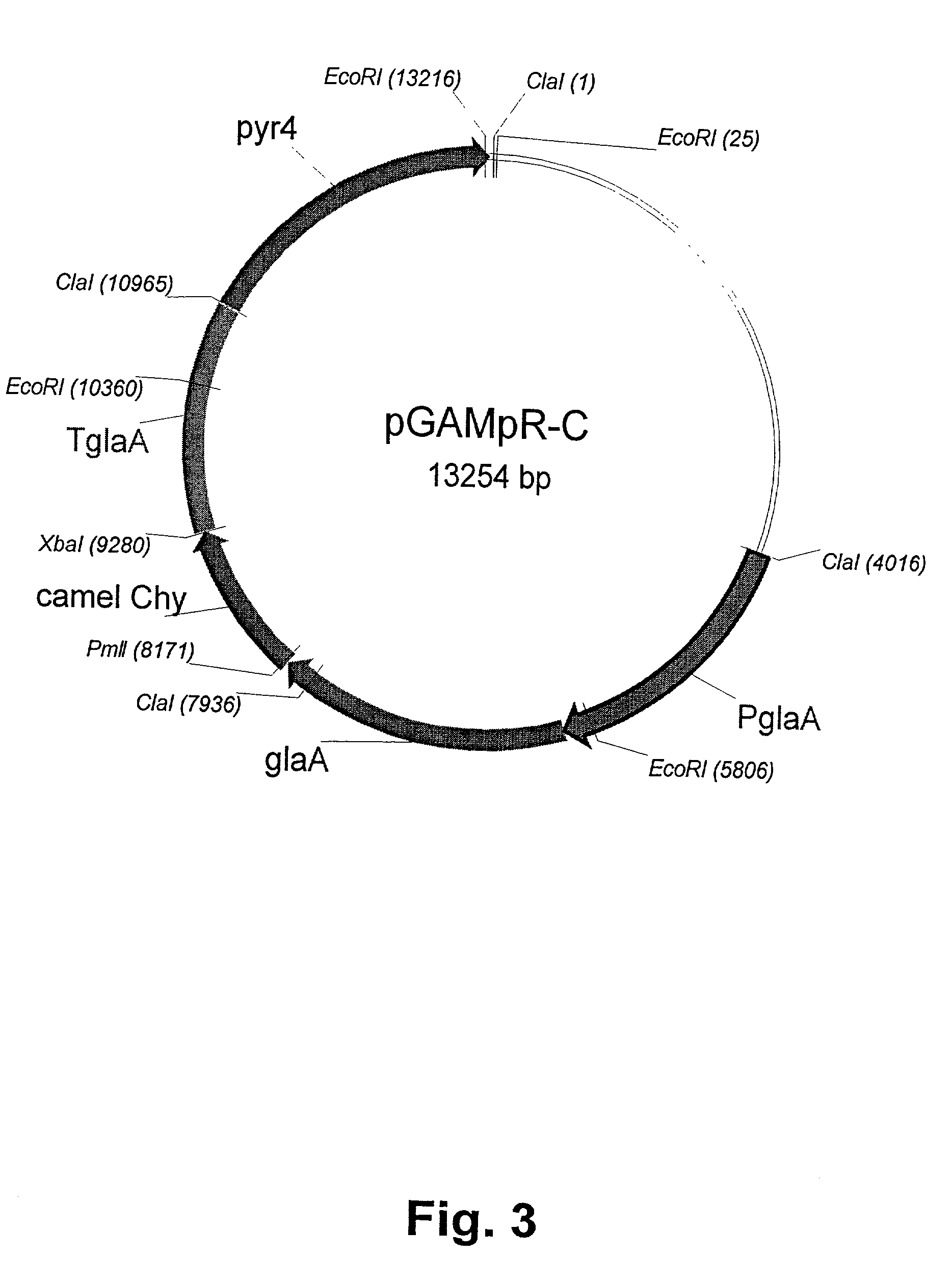
[0080] Transformation of Aspergillus niger var. awamori with pGAMpR-C
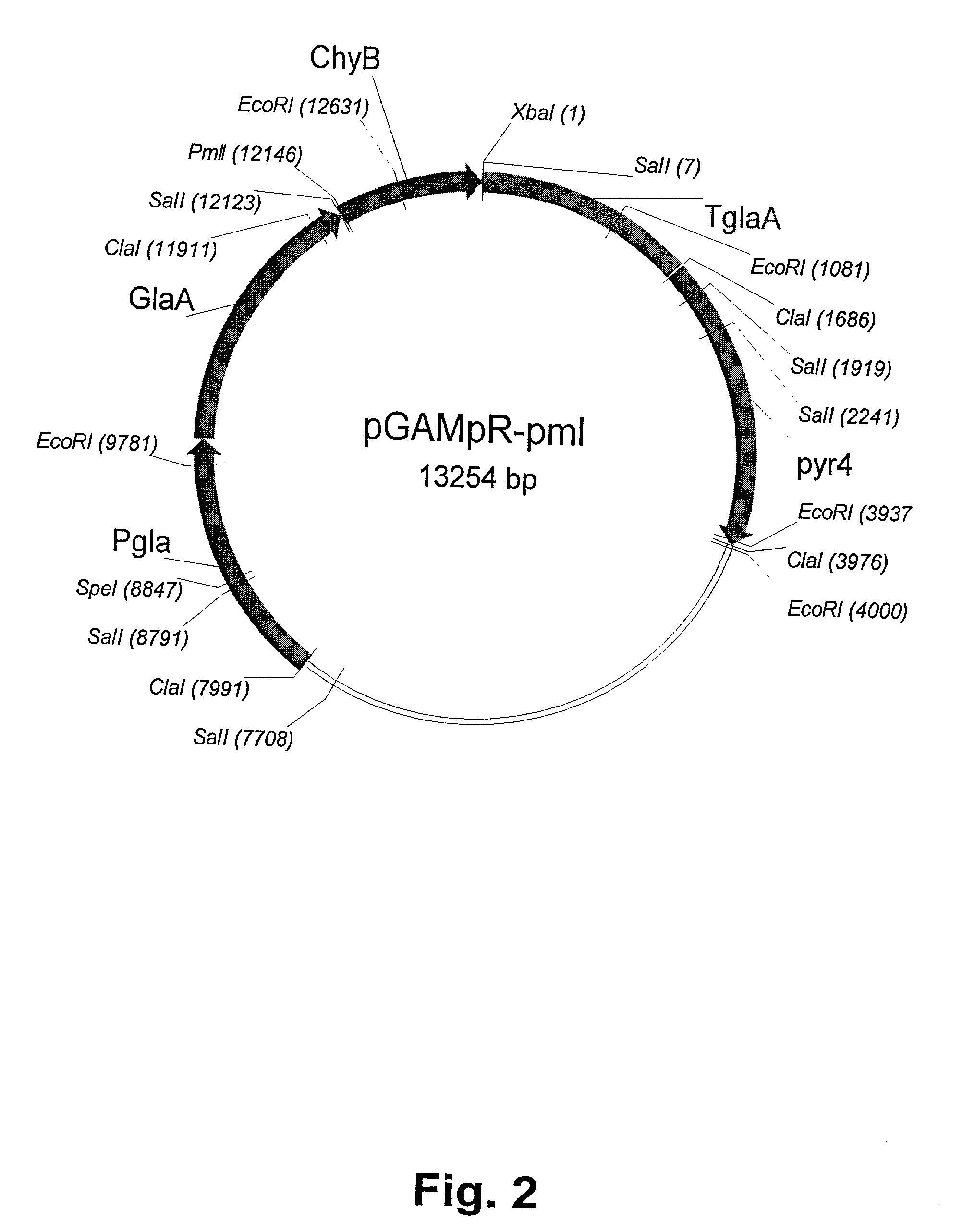
[0081] For these transformation experiments, a derivative of Aspergillus niger var. awamori, strain dgr246pyrG (Ward et al., 1993) was used as recipient. This strain is a derivative of Aspergillus niger var. awamori strain GCl-HF1-2dgr246 having a pyrG mutation, rendering the strain incapable of growing in the absence of uridine, and which comprises several copies of the pGAMpR plasmid. The derivative strain, dgr246pyrG used as recipient is derived by curing the pyrG mutant parent strain for all copies of pGAMpR.
[0082] The dgr246pyrG strain has been deposited under the Budapest Treaty with the Centraalbureau voor Schimmelcultures (CBS), Oosterstraat 1, P.O. Box 273, 3740 AG Baarn, The Netherlands, on Jun. 13, 2000 under the accession No. CBS 108914.
[0083] An optimised protocol as developed by Chr. Hansen A / S was applied for transformation of the "cured" Aspergillus strain. This protocol comprises the steps of provi...
example 3
[0095] Production of camel chymosin using recombinant Aspergillus niger var. awamori
[0096] 3.1 Selection of transformants producing high amounts of chymosin
[0097] To select the transformants that produced the most chymosin, a small scale (20 ml) shake flask experiments with 45 transformants was carried out. 20 ml of CSL medium (see above) was inoculated with 1.times.10.sup.6 spores of each transformant, incubation 24-48 hrs at 37.degree. C. and 200 rpm. 2 ml of these precultures was used for inoculation of 20 ml medium followed by incubation for 10 days at 37.degree. C., 200 rpm. After incubation the cultures were centrifuged at 14,000 rpm using an Eppendorf centrifuge and the clear supernatants were collected and stored at -20.degree. C. until determination of chymosin activity. As controls, both the recipient strain and an Aspergillus niger var. awamori production strain for bovine chymosin, dgr246chlor25 (Dunn-Coleman et al., 1991) containing the pGAMpR (spores of this strain use...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com