Benzylpiperazinyl-indolinylethanones
- Summary
- Abstract
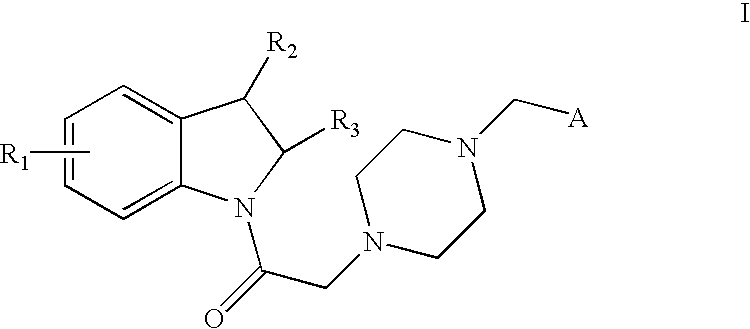
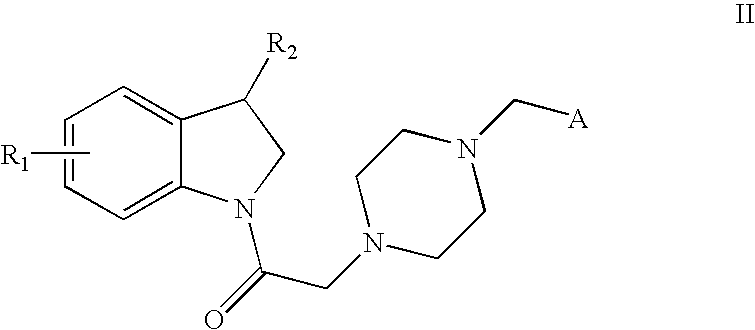
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
2-{4-[(4-Chlorophenyl)methyl]piperazinyl}-1-(3-methylindolinyl)ethan-1-one
Part A: 3-Methylindoline
[0095] 17
[0096] Hydrochloric acid (10.5 N, 6 mL) is added dropwise to a stirred mixture of 3-methylindole (3.93 g, 30 mmol) and trimethylamine-borane (8.75 g, 120 mmol) in room temperature dioxane (30 mL). The mixture is then heated at reflux for about 30 min. After cooling to room temperature, 6 N hydrochloric acid (24 mL) is carefully added and the mixture is refluxed for 15 min. Most of the dioxane is then removed under reduced pressure, and the residue is diluted with water (200 mL). Some insoluble material is extracted with ether, and the aqueous solution is basified with 30% aqueous sodium hydroxide to pH.about.10. The oily product is extracted with ether, the extracts are washed with water and brine, dried over anhydrous MgSO.sub.4, filtered and evaporated under reduced pressure to provide the product as a light yellow oil (3.75 g, 94% yield).
[0097] .sup.1HNMR (CDCl.sub.3) .delta...
example 2
1-((2R,3R)-2,3-Dimethylindolinyl)-2-{4-[(4-Chlorophenyl)methyl]piperazinyl- }ethan-1-one, and
1-((2S,3S)-2,3-Dimethylindolinyl)-2-{4-[(4-Chlorophenyl)methyl]piperazinyl- }ethan-1-one
Part A: cis- and trans-2,3-Dimethylindolines
[0103] 20
[0104] These two indolines are prepared starting from 2,3-dimethylindole according to a literature procedure. (Synthesis, 508, (Berger, 1974)).
Part B: 2-Chloro-1-(cis-2,3-Dimethylindolinyl)ethan-1-one
[0105] 21
[0106] The title compound is prepared by the procedure as described in Example 1 (part B) to provide a colorless solid in 75% yield. mp 83-84.degree. C.; .sup.1HNMR (CDCl.sub.3) .delta.8.17 (m, 1H), 7.28-7.08 (m, 7H), 4.24 (m, 1H), 4.18 (s, 2H), 2.90 (m, 1H), 1.32 (d, J=6.3 Hz, 3H), 1.25 (d, J=6.9 Hz, 3H); LC-MS (APCI, m / z) 224 (M+1).sup.+.
Part C: 1-((2R,3R)-2,3-Dimethylindolinyl)-2-{4-[(4-Chlorophenyl)methyl]pip- erazinyl}ethan-1-one and
1-((2S,3S)-2,3-Dimethylindolinyl)-2-{4-[(4-Chlorophenyl)methyl]piperazinyl- }ethan-1-one
[0107] 22
[0108] These tw...
example 3
Methyl (2S)-1-(2-{4-[(4-chlorophenyl)methyl]-piperazinyl}indoline-2-carbox- ylate
Part A: Methyl (2S)-1-(2-chloroacetyl)indoline-2-carboxylate
[0109] 23
[0110] The title compound is prepared starting from methyl (2S)-1-(2-chloroacetyl)indoline-2-carboxylate monohydrate (J. Org. Chem. 62, Bertini Gross, 7679 (1997)) and chloroacetyl chloride by the procedure described in Example 1 (step B, 3 eq. triethylamine is used) to provide the product as a colorless oil in 89% yield. .sup.1HNMR (CDCl.sub.3) .delta.7.29-7.06 (m, 4H), 5.16 (m, 1H), 4.12 (s, 2H), 3.77 (s, 3H), 3.64 (m, 1H), 3.41-3.35 (m, 1H); LC-MS (APCI, m / z) 254 (M+1).sup.+.
Part B: Methyl (2S)-1-(2-{4-[(4-chlorophenyl)methyl]piperazinyl}indoline-2- -carboxylate
[0111] 24
[0112] The title compound is prepared starting from methyl (2S)-1-(2-chloroacetyl)indoline-2-carboxylate and [(4-chlorophenyl)methyl- ]piperazine by the procedure described in Example 1 (Part C), and purified by crystallization from ethyl acetate and hexanes to provi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com