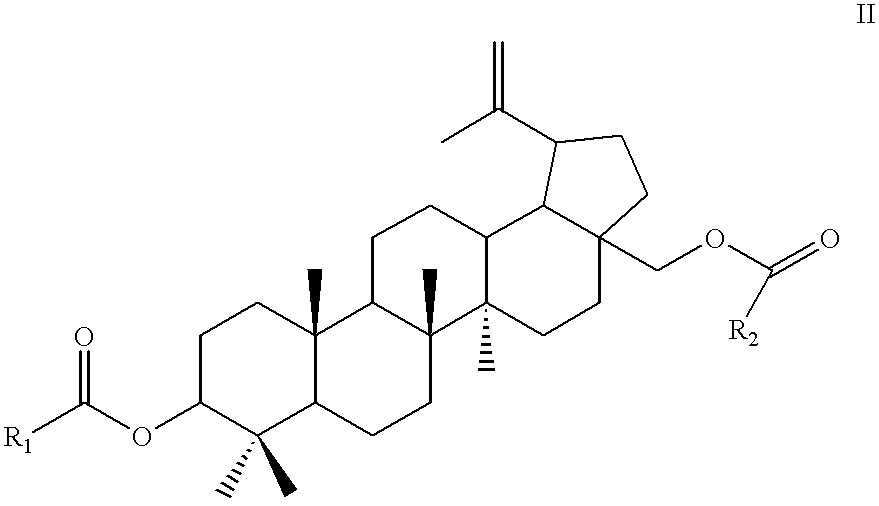
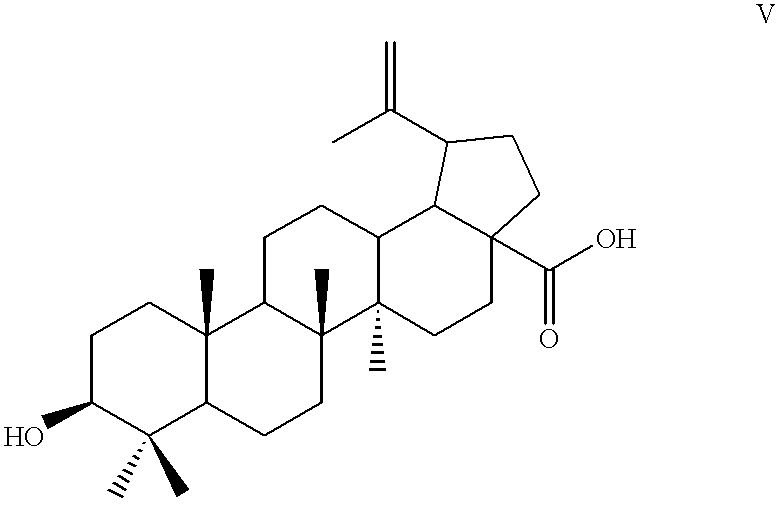
Method for manufacturing betulinic acid
a manufacturing method and technology for betulinic acid, applied in the field of betulinic acid manufacturing, can solve the problems of unsatisfactory commercial scale (e.g., kilogram) production of betulinic acid, unfavorable disclosure of purification steps, and inability to meet the requirements of a commercial scale, etc., to achieve less toxic, less time, and less expensive
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
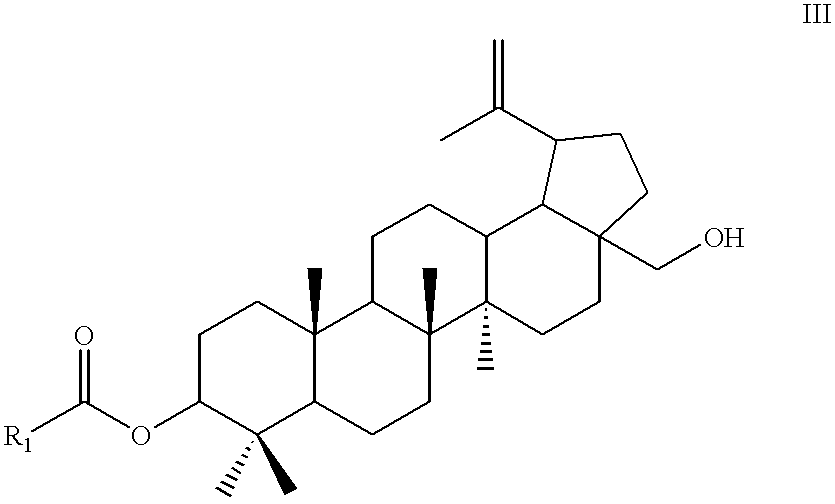
[0065] Betulin-3-acetate (III) 9
[0066] Acetic anhydride (85 ml) and acetic acid (800 ml) were introduced into round bottom flask (3 L). Betulin (I) (100 g, 0.2259 mol) was added to the stirred solution and refluxed for 3 hours. The reaction mixture was allowed to cool to 50.degree. C. and acetic acid was evaporated under reduced pressure (25-30 mm Hg). Cream-white crystals of intermediate betulin-3,28-diacetate (II) were obtained after storage in vacuo (118.8 g, 0.2257 mol, 50-60.degree. C., 0.1 mm Hg).
[0067] Isopropanol (i.e., i-PrOH) (2.5 L) and powdered aluminum iso-propoxide (i.e., Al(O-i-Pr).sub.3) (100 g, 0.223 mol) were added to the flask and the mixture was refluxed for 1.5 hours. Isopropyl alcohol was then removed under reduced pressure (100 mm Hg) at 30-33.degree. C. The resulting white-orange crystals were dissolved in dichloromethane (1 L) and water (60 ml) was added to the solution. After stirring (10-15 minutes) the precipitated material was filtered, extracted with di...
example 2
[0068] Betulinic aldehyde-3-acetate (VI) 10
[0069] Oxalyl chloride (15.72 g, 0.1237 mol) in methylene chloride (i.e., CH.sub.2Cl.sub.2) (500 ml) was placed in a round bottom two-neck flask (2 L). After cooling to -50 to -60.degree. C. (dry ice bath) and with efficient stirring was added dimethylsulfoxide (i.e., DMSO) (12.87 g, 0.165 mol) in methylene chloride (500 ml) drop-wise over 5 to 10 minutes. The mixture was stirred for an additional 5 to 10 minutes (until gas evolution stops). Betulin-3-acetate (III) (powdered) (0.0825 mol, 40 g) was then added and allowed to stand for 45 minutes. After triethylamine (41.67 g, 0.4126 mol) was added, the cooling bath was removed and temperature allowed to increase to 10.degree. C. Cold water (200 ml) was added and the mixture was extracted with methylene chloride (3.times.100 ml). The combined organic extracts were washed with water (5.times.100 ml), 5% HCl (2.times.100 ml) and brine (2.times.100 ml). After drying over sodium sulfate (10 g), e...
example 3
[0070] Betulinic aldehyde-3-acetate (VI) 11
[0071] Oxalyl chloride (7.86 g, 62 mmol) in trifluoromethylbenzene (250 ml) was placed in a round bottom two-neck flask (2 L). After cooling to -30 to -35.degree. C. (i-PrOH-dry ice bath) and with efficient stirring was added dimethylsulfoxide (6.44 g, 83 mmol) in trifluoromethylbenzene (250 ml) drop-wise over 5 to 10 minutes. The mixture was stirred for an additional 5-10 minutes (until gas evolution stops). Powdered 3-O-acetyl-betulin (III) (40 g, 41 mmol) was then added. The resulting mixture was allowed to stand for 45 minutes. After triethylamine (41.67 g, 206 mmol) was added, the cooling bath was removed and the temperature was allowed to increase to 10.degree. C. Cold water (100 ml) was added and the mixture was extracted with trifluoromethylbenzene (3.times.50 ml). The combined organic extracts were washed with water (5.times.50 ml), 5% HCl (2.times.50 ml) with brine (2.times.50 ml). After drying over sodium sulfate (5 g), evaporati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com