Bunte salts from amino acids and oligopeptides as protective ingredients in hair treatment means
a technology of amino acids and oligopeptides, which is applied in the field of hair treatment agents, can solve the problems of inability to achieve a long-term nurturing effect, damage to hair, and oxidative damage to the remaining structural components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Formulations
The following formulations were created (all data in wt. %)
1.1. Pre-Treatment Agent (VM)
[0650]
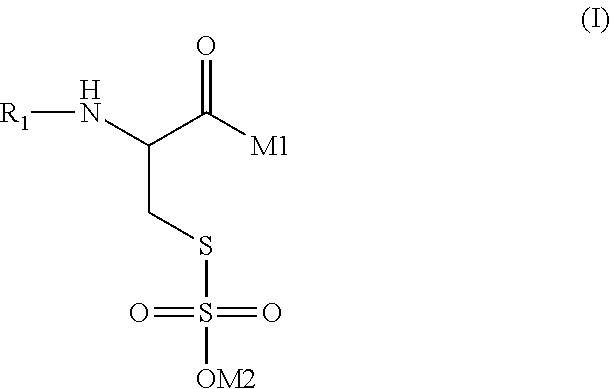
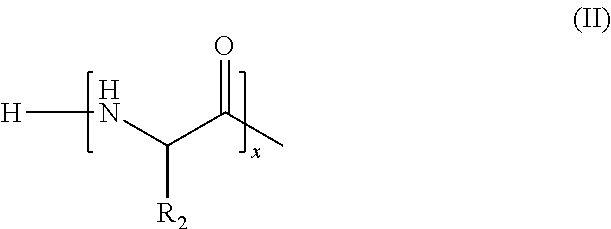
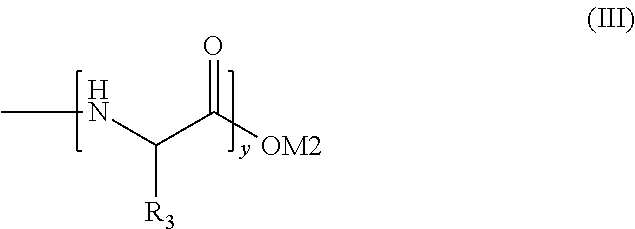
VM1VM2VM3Citric acid (anhydrous)0.5Sodium lauryl ether sulfate50.050.050.0(25% hydrous solution)Disodium dicocoatepodicate7.07.07.0Salicylic acid0.20.20.2Sodium benzoate0.50.50.5Euperlan PK 300 AM2.02.02.0(approx. 60-64% solids; INCI: glycoldistearate, glycerine, laureth-4,cocamidopropyl betaine (cognis))Cetiol HE1.01.01.0(coconut glyceride with approx. 7.3 EO units(INCI: PEG-7 glyceryl cocoate) (cognis))Oligopeptide#0.010.11.0Polyquaternium-100.50.50.5PEG-40 Hydrogenated Castor Oil1.01.01.0Sodium chloride0.50.50.5Waterad 100ad 100ad 100#Oligopeptide mixture of formula (I), where R1 = formula (II), M1 = formula (III), x = 1-10 and y = 1-10, M2 = hydrogen, average molecular weight from about 400-about 800 Dalton
[0651]
VM4VM5VM6Citric acid (anhydrous)0.50.50.5Sodium lauryl ether sulfate50.050.050.0(25% hydrous solution)Disodium dicocoatepodicate7.07.07.0Salicylic acid0.20.20.2So...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wt. % | aaaaa | aaaaa |
| molecular weight Mw | aaaaa | aaaaa |
| molecular weight Mw | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com