Nanostructures for modulating intercellular communication and uses thereof
a technology of nanostructures and compositions, applied in the direction of pharmaceutical non-active ingredients, pharmaceutical active ingredients, organic active ingredients, etc., can solve the problems of significant limitations of in vivo applications and variability of methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0267]The present example demonstrates the inhibition of intercellular communication by contacting cells with synthetic nanostructure. As further described below, HDL NPs target SR-B1, manipulate cellular cholesterol homeostasis, and modulate the uptake of exosomes by disrupting lipid rafts.
[0268]HDL NP Synthesis:
[0269]Biomimetic high-density lipoprotein-like nanoparticles (HDL NPs) were synthesized and characterized as previously described [Yang et al. 2013; Luthi et al. 2012; Thaxton et al. 2009]. Briefly, citrate stabilized 5 nm diameter gold nanoparticles (AuNP, Ted Pella) were used as a template for surface chemical modification. Purified human apolipoprotein AI (apoA-I) was incubated with a solution of AuNPs (80 nM) at 5-fold molar excess (400 nM, final) for 1 hour at room temperature (RT) with gentle stirring. Next, the phospholipids, 1-2-dipalmitoyl-sn-glycero-3-phosphocholine and 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate] were added a...
example 2
[0305]The present example demonstrates the preparation of synthetic nanostructures with an agent and without an agent and the loading of vesicles with these nanostructures.
[0306]Synthesis of HDL NPs with and without Agents:
[0307]HDL NPs were synthesized using 5 nm citrate stabilized colloidal gold nanoparticles (BBI Solutions) incubated with five-fold molar excess human apolipoprotein AI (Meridian Life Sciences) for one hour with shaking. The phospholipid 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[3-(2-pyridyldithio)propionate] (DPPTE, Avanti Polar Lipids) was dissolved in ethanol and added in 250-fold molar excess to gold. Other lipids varied depending on the type of particle.
[0308]For particles without tracer, 250-fold molar excess to gold 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, Avanti) dissolved in ethanol was added and allowed to incubate overnight with shaking.
[0309]For particles with a rhodamine tracer, 200-fold molar excess to gold DPPC dissolved in ethano...
example 3
[0315]The present example demonstrates that identifying and targeting a natural cellular pathway of exosome production provides a new mechanism for efficient and stable manipulation of exosomes that may enable in vivo, ex vivo, or in vitro applications.
[0316]Synthesis of Rh-HDL NPs:
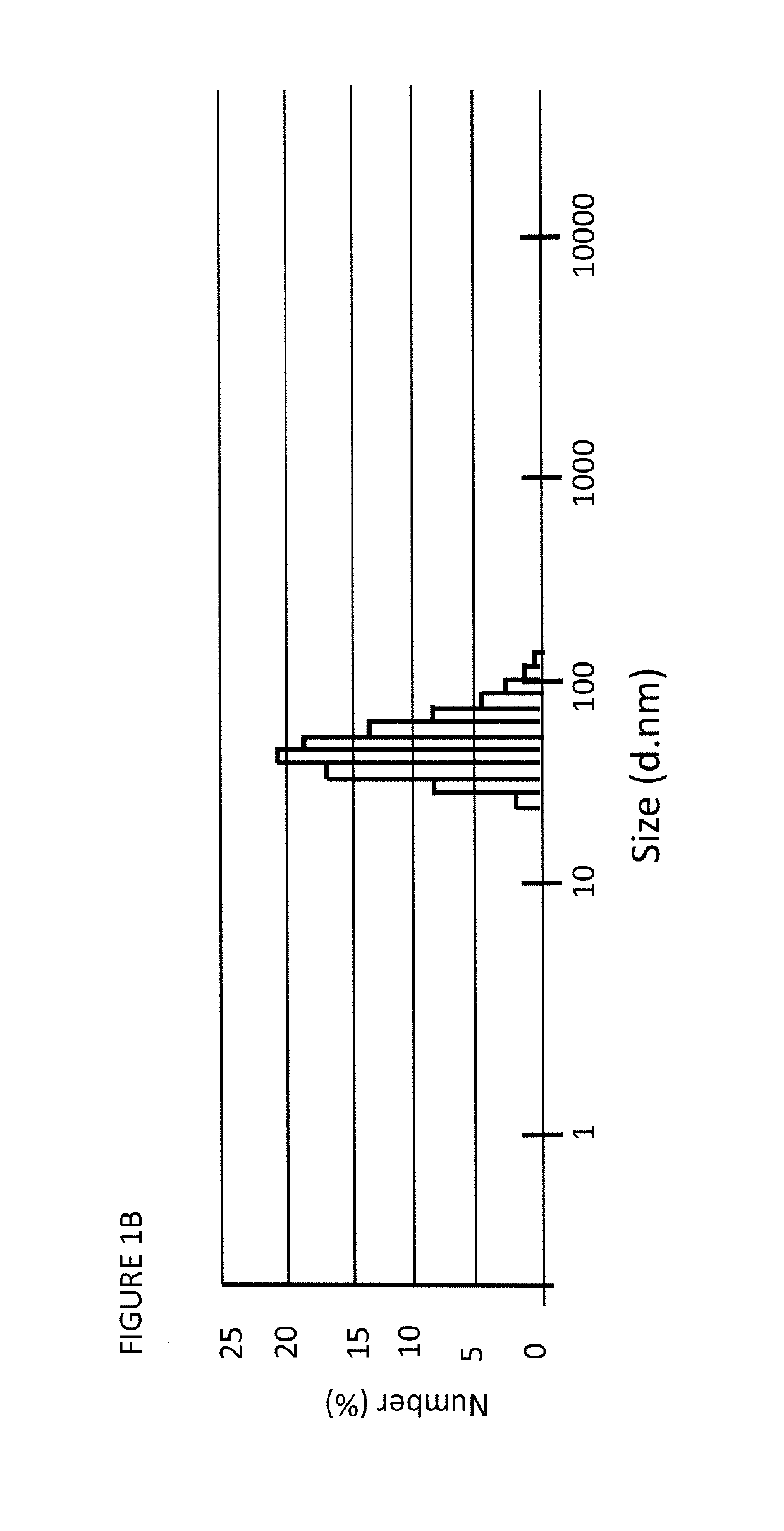
[0317]We synthesized HDL NPs with rhodamine-labeled fluorescent phospholipids (Rh-HDL NP, see below). Data demonstrate that there are ˜17 fluorescent phospholipids on each Rh-HDL NP (Table 1). Also, incorporation of the rhodamine fluorophore was evident by a noticeable absorption peak at 560 nm (FIG. 22). The Rh-HDL NPs have physical properties similar to those of HDL NPs that lack the labeled phospholipid (Table 1).
[0318]
TABLE 1Physical characterization data for Rh-HDL NPs.HDL NPRh-HDL NPSize (nm)13.64 ± 1.2312.85 ± 0.44UV-Vis λmax (nM)519517Zeta Potential (mV)−41.6 ± 0.71−37.9 ± 2.12Rhodamine lipids / particle 0 17
[0319]Characterization of Rh-HDL NP Containing Exosomes:
[0320]We initiated our studies with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com