Process for producing 1-oxacephalosporin-7alpha-methoxy-3-chloromethyl derivative
A production method, methyl technology, applied in the field of intermediates for the synthesis of 1-oxacephalosporins, which can solve the problems of unsatisfactory yield, unrecognizable, low yield, etc.
Inactive Publication Date: 2007-06-13
SHIONOGI & CO LTD
View PDF3 Cites 16 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In Example 4, intermediate 6 was synthesized by reacting 3-exomethylene compound 1 with chlorine in the presence of quinoline as a base, however, the yield was as low as about 9.6%. In Example 5 , the chlorination was carried out using α-picoline as a base, however, optical reactions were also used, and the yields were still not satisfactory
In the step from intermediate 6 to compound 4, NaOMe / MeOH was used as methoxylating agent, however column chromatography was also used to remove by-products, therefore, this method also cannot be regarded as industrially favorable
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0011]
Embodiment 2
[0012]
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
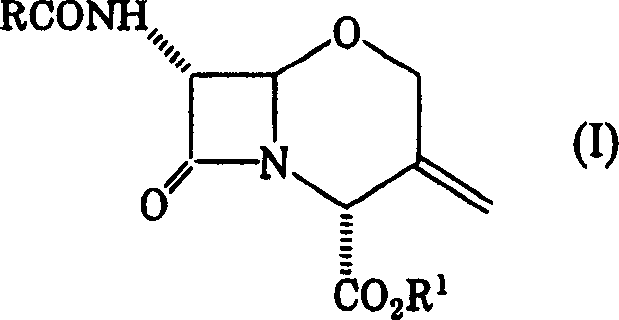
To provide a novel production method of oxacephem compound. [Solution] A method of producing Compound (IV) shown by the formula: (wherein R represents an acyl residue; R 1 represents carboxy protecting group; Me represents methyl and X represents halogen), the method comprising the steps of: (First step) letting Compound (I) shown by Formula: (wherein, R represents an acyl residue; R 1 represents carboxy protecting group) react with a halogenating agent in the presence of a base; (Second step) adding MOMe (M represents alkaline metal; Me represents methyl) in the presence of a halogenating agent after completion of the first step; and (Third step) adding a reducing agent after completion of the second step.
Description
technical field [0001] The present invention relates to a production method of an intermediate for the synthesis of 1-oxacephalosporin which can be used as an antimicrobial agent. Background technique [0002] 1-Oxacephalosporin-7α-methoxy- 3-Chloromethyl derivatives (hereinafter also simply referred to as 7α-methoxy-3-chloromethyl compounds) are known. As a production method thereof, there is known a method in which a raw material 3-exomethylene compound is added with Cl 2 7α-Methoxylation then occurs by light irradiation. However, photoirradiation generally requires expensive optical reaction equipment and thus is not industrially advantageous. For example, Non-Patent Document 1 describes the following reaction. [chemical formula 1] Non-Patent Documents 2 and 3 describe the following methods. [chemical formula 2] On the other hand, Patent Document 1 describes a production method that avoids optical reactions. The outline of this reaction is as follo...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D505/06C07D505/18C07D498/04C07D505/00
CPCC07D505/00Y02P20/55
Inventor 鸿池敏郎
Owner SHIONOGI & CO LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com