Metabolites of selective androgen receptor modulators and methods of use thereof
A technology of androgen receptors and metabolites, applied in the field of selective androgen receptor modulators, which can solve problems such as harm to individual health, increased susceptibility to infection, and susceptibility to damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0334] The preparation of pharmaceutical compositions containing active ingredients is known in the art, for example by mixing, granulating or tabletting methods. The active therapeutic ingredient is usually mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. For oral administration, the SARM drugs or their physiologically tolerable derivatives such as salts, esters, N-oxides etc. are mixed with additives customary for this purpose such as excipients, stabilizers or inert diluents, and Conversion into a form suitable for administration, such as tablets, coated tablets, hard or soft gelatin capsules, aqueous, alcoholic or oily solutions, is carried out by conventional methods. For parenteral administration, the SARM drugs or their physiologically tolerable derivatives such as salts, esters, N-oxides, etc. are converted into solutions, suspensions or emulsions, if necessary, with conventionally used and suitable Substances of i...
Embodiment 1
[0342] Non-steroidal ligands with androgenic and anabolic activity
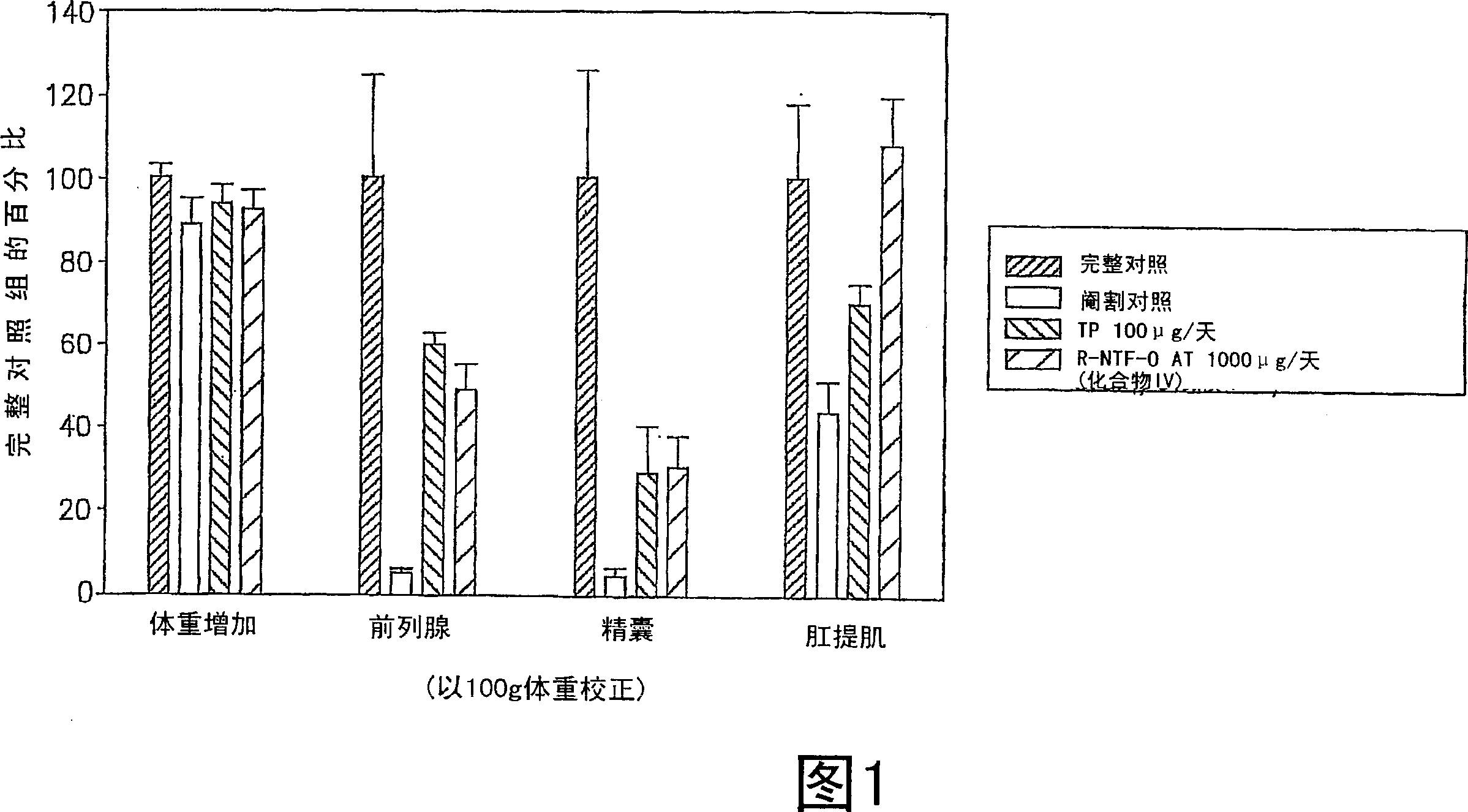
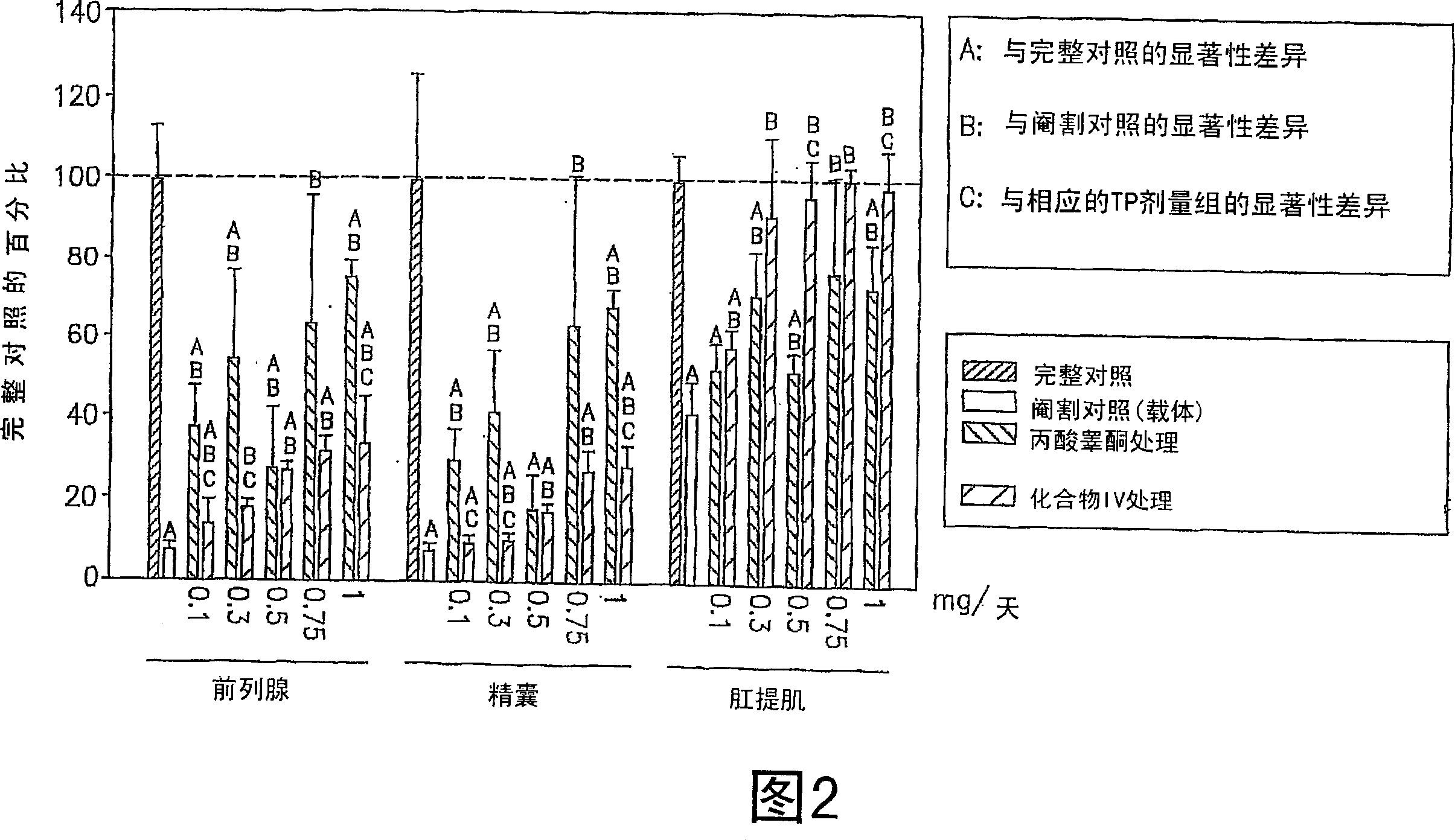
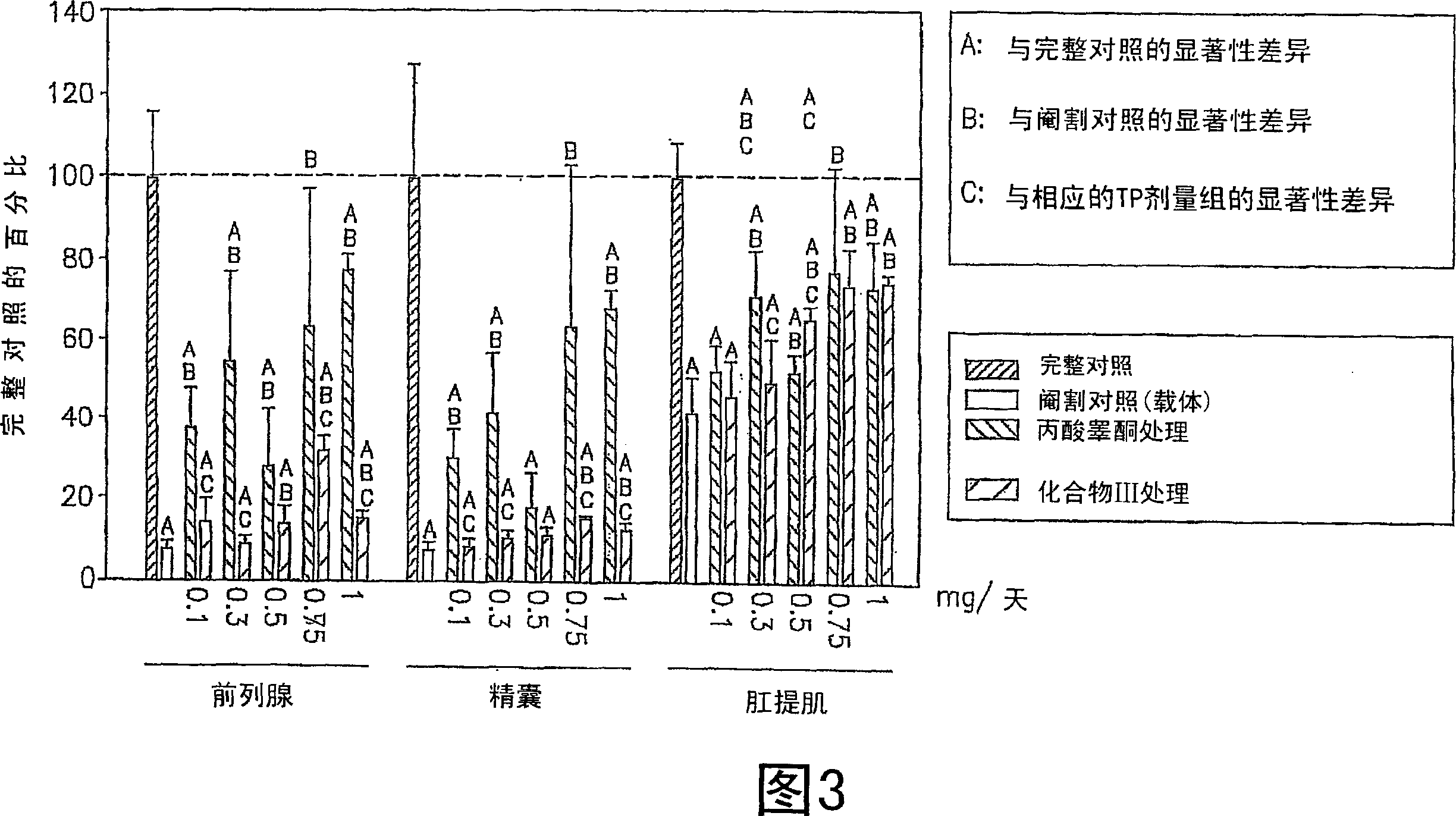
[0343] Some of the SARM compounds provided herein were designed and synthesized and evaluated for their in vitro and in vivo pharmacological activities. Study of androgen receptor binding affinity in vitro and ability to maintain androgen-dependent tissue growth in castrated animals. Androgenic activity is monitored by the ability of the SARM compound to maintain and / or stimulate the growth of the prostate and seminal vesicles, as measured by body weight. Anabolic activity is monitored as the ability of the SARM compound to maintain and / or stimulate growth of the levator ani muscle as measured by weight.
[0344] resolve resolution
[0345] (2R)-1-Methacryloylpyrrolidine-2-carboxylic acid (R-129). D-proline (R-128, 14.93 g, 0.13 mol) was dissolved in 71 mL of 2N NaOH, cooled on an ice bath, and the resulting basic solution was diluted with acetone (71 mL). A solution of methacryloyl chloride 127 (13.56 g,...
Embodiment 2
[0354] Non-steroidal ligands with androgenic and anabolic activity
[0355] The in vivo efficacy and acute toxicity of four new non-steroidal androgens (compounds III, IV, VI and VII) were tested in rats. In vitro assays confirm that these compounds bind the androgen receptor with very high affinity. The structures and names of the four compounds are shown below:
[0356]
[0357] Compound III R=F
[0358] Compound IV R=NHCOCH 3
[0359] Compound VI R=COCH 3
[0360] Compound VII R=COC 2 h 5
[0361] experimental method
[0362]Materials: S isomers of compounds III, IV, VI and VII and R isomers of compound III were synthesized according to the route shown in FIG. 9 . Testosterone propionate (TP), polyethylene glycol 300 (PEG300, reagent grade) and neutral buffered formalin (10% w / v) were purchased from Sigma Chemical Company (St Louis, MO). Alzet osmotic pumps (model 2002) were purchased from AlzaCorp. (Palo Alto, CA).
[0363] Animals: Juvenile male Sprague-Daw...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com