Beta-carboline alkaloid, their preparation method and use
A technology of alkaloids and carbolines, which is applied in the treatment and prevention of AIDS. In the field of β-carboline alkaloids, it can solve the problems of no anti-HIV-1 activity of β-carboline alkaloids, and achieve good anti-HIV- 1 The effect of activity and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
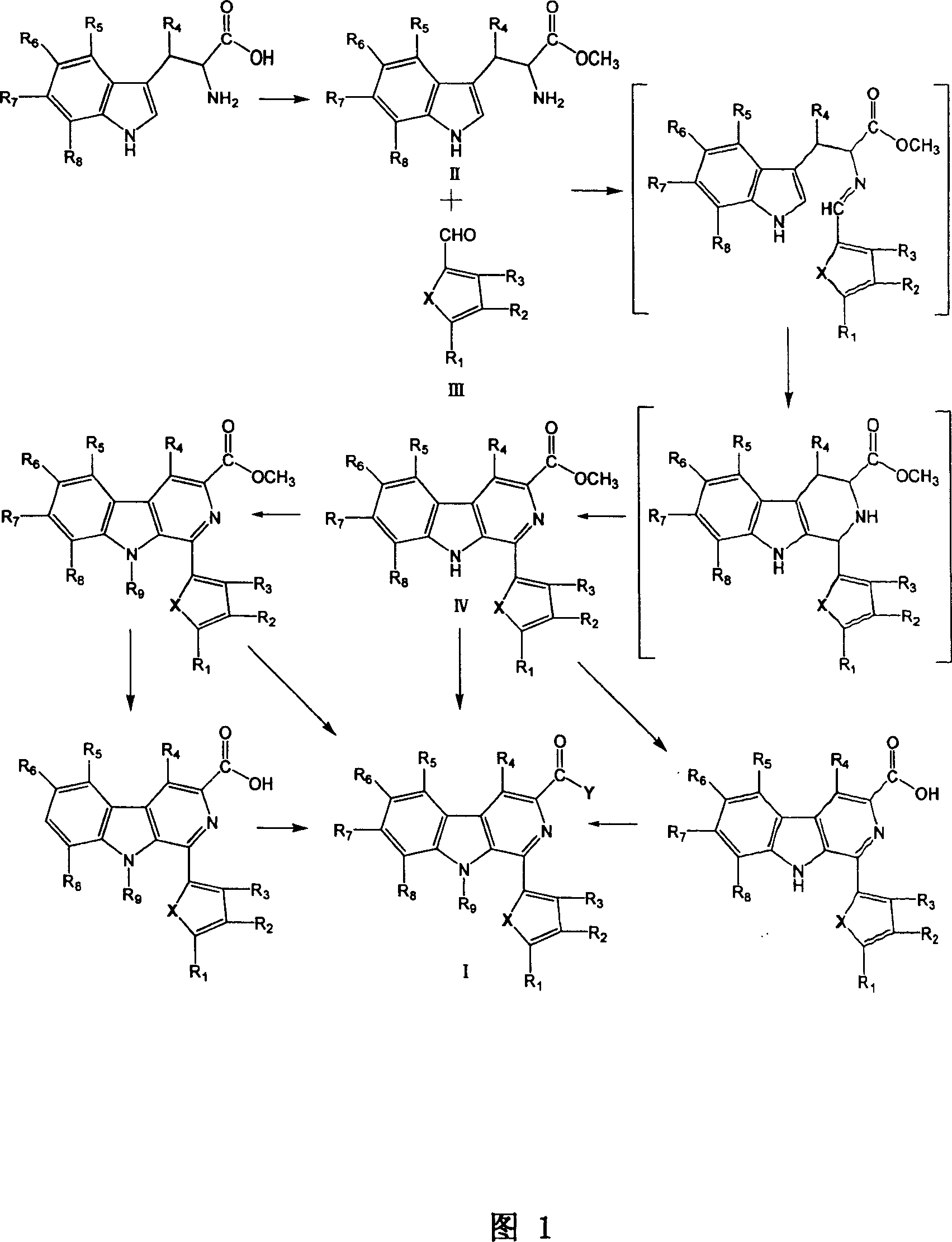
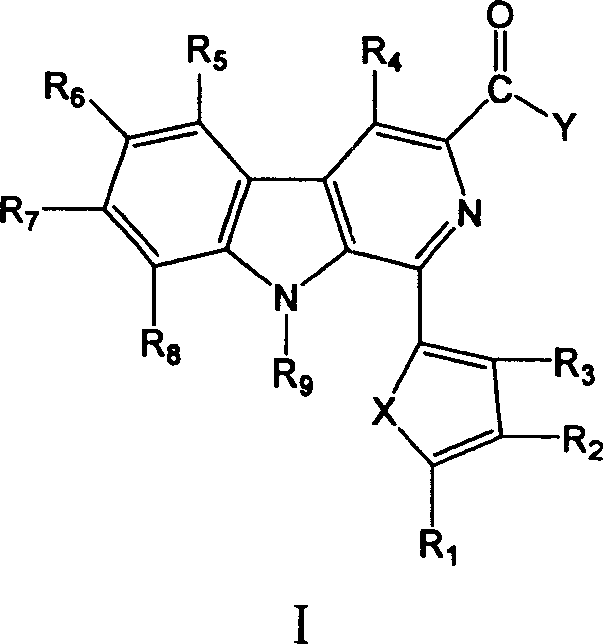
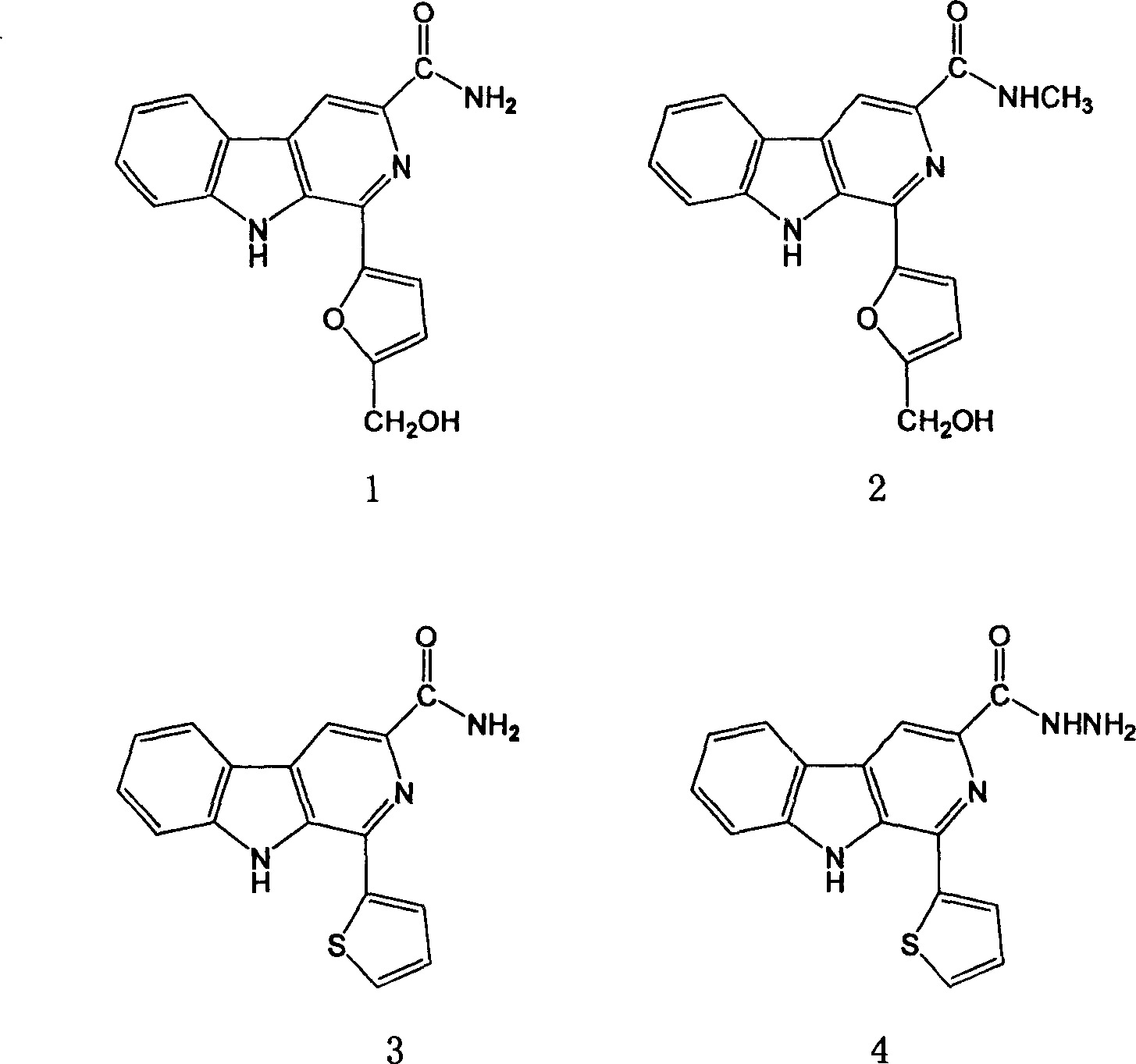
[0051] Embodiment 1: Preparation 1-(5'-hydroxymethyl-2-furan)-β-carboline-3-formamide (compound 1)
[0052] Step A: Synthesis of L-Tryptophan Methyl Ester
[0053] Add 200mL dry methanol to 10mmol L-tryptophan, cool to below -10°C under magnetic stirring, slowly add 10mL thionyl chloride, then allow to rise to room temperature naturally, and then heat to reflux for 3h. Spin off excess methanol and thionyl chloride under reduced pressure, then add an appropriate amount of water to dissolve, adjust the pH value to 9-10, extract with ethyl acetate, combine the organic phases, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure, and obtain a white Solid 2.13g, Yield: 98%, m.p.87-90℃.C 12 h 14 N 2 o 2 .EI-MS m / z: 218(15%), 159(10%), 130(100%), 77(13%). 1 H NMR (500MHz, CDCl 3 )δ (ppm): 8.27 (1H, s, br), 7.61 (1H, d, J = 7.8Hz), 7.35 (1H, d, J = 8.0Hz), 7.19 (1H, t, J = 7.4Hz) , 7.12(1H, t, J=7.4Hz), 7.05(1H, s), 3.83(1H, m), 3.71(3H, s), 3.28(1H, ...
Embodiment 2
[0058] Embodiment 2: Preparation of N-methyl-1-(5'-hydroxymethyl-2-furan)-β-carboline-3-formamide (compound 2)
[0059] Add 20 mL of methanol to 1 mmol of 1-(5'-acetoxymethyl-2-furan)-β-carboline-3-carboxylate, and add 4 mL of methylamine (33% methylamine in ethanol) under magnetic stirring , heated to 50°C, TLC showed that the raw material disappeared, the solvent was evaporated under reduced pressure, and column chromatography gave 193.2 mg of a yellow solid, Yield: 61%, mp241-314°C, C 18 h 15 N 3 o 3 .FAB + -MS m / z: 322(M+1). 1 H NMR (500MHz, DMSO-d 6 )δ (ppm): 11.52 (1H, s), 8.72 (1H, s), 8.39 (1H, d, J = 8.0Hz), 7.82 (1H, d, J = 8.0Hz), 7.68 (1H, d, J = 2.7Hz), 7.61 (2H, t, J = 8.5Hz), 7.31 (1H, t, J = 7, 5Hz), 6.62 (1H, d, J = 3.0Hz), 4.68 (2H, s), 2.90(3H,s). 13 C NMR (100MHz, DMSO-d 6 )δ (ppm): 164.9, 157.2, 151.7, 141.4, 139.6, 131.4, 130.2, 128.7, 128.6, 122.0, 121.0, 120.2, 112.7, 112.2, 111.0, 109.1, 55.9.
Embodiment 3
[0060] Example 3 Preparation of 1-(2-thiophene)-β-carboline-3-formamide (compound 3)
[0061] Step A: Synthesis of methyl 1-(2-thiophene)-β-carboline-3-carboxylate
[0062] Using 2-thiophene aldehyde and L-tryptophan methyl ester as raw materials, the operation is similar to step B of Example 1 to obtain 2.61 g of light yellow solid, Yield: 85%, mp151-154 °C, C 17 h 12 N 2 o 2 S.FAB + -MS m / z: 309(M+1). 1 HNMR (400MHz, CDCl 3 )δ (ppm): 8.75 (1H, s), 8.15 (1H, d, J = 8.0Hz), 7.82 (1H, d, J = 8.0Hz), 7.58 (2H, m), 7.47 (1H, d, J=4.0Hz), 7.33(1H, m), 7.16(1H, d, J=4.0Hz), 4.05(3H, s). 13 CNMR (100MHz, CDCl 3 )δ (ppm) 166.6, 141.6, 140.9, 136.9, 133.6, 130.0, 128.7, 127.8, 127.7, 127.6, 126.0, 121.6, 121.4, 120.8, 116.5, 112.2, 52.5.
[0063] Step B: Synthesis of 1-(2-thiophene)-β-carboline-3-carboxamide
[0064] Using methyl 1-(2-thiophene)-β-carboline-3-carboxylate as raw material, the operation was similar to Step C of Example 1 to obtain 205.4 mg of yellow solid, Yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com