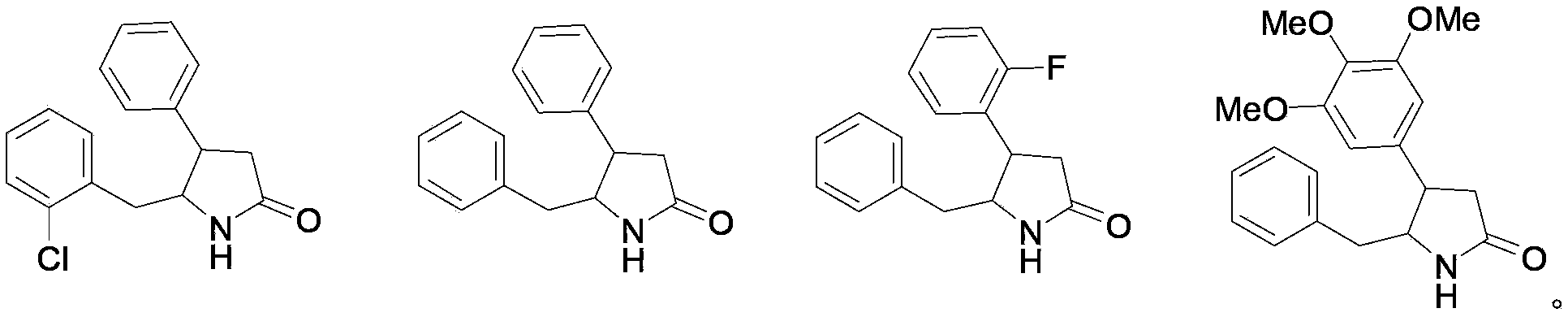
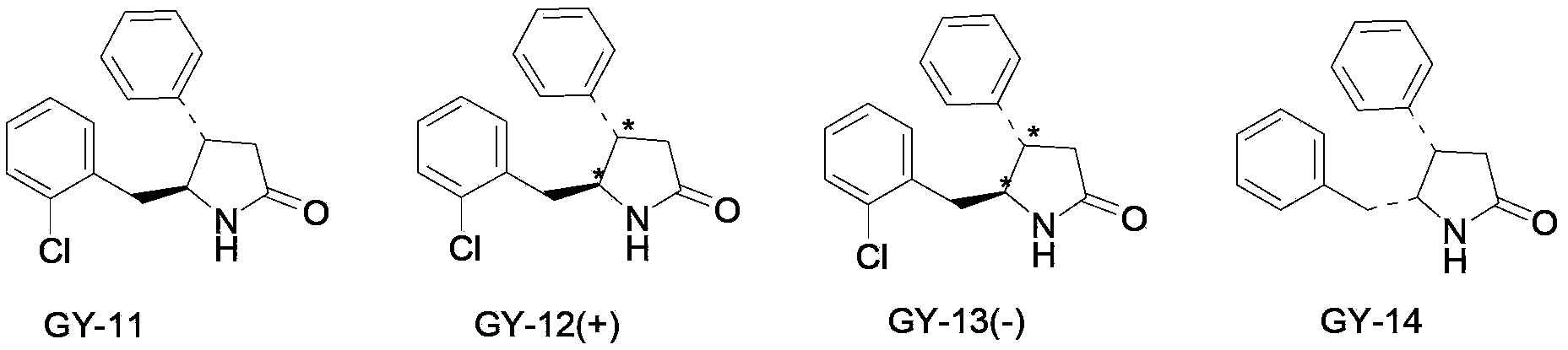
New use of 4, 5-disubstituted-2-pyrrolidone compound
A technology of pyrrolidone and compound, applied in the field of pharmaceutical application, can solve the problems of forgetfulness, toxic and side effects, bone marrow suppression and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 4,5-disubstituted-2-pyrrolidone compound (ie general formula I):
[0039] The preparation of trans 4-phenyl-5-o-chlorobenzyl-2-pyrrolidone is according to the invention patent ZL97123419.1 (d1-trans-4-phenyl-5-o-chlorobenzylpyrrolidone-2 synthesis process) The disclosed method, the synthetic route is as follows:
[0040]
[0041] (1) Synthesis of 2-o-chlorophenylnitroethylene (intermediate IV)
[0042] Method A: Mix 8g of o-chlorobenzaldehyde (0.057mol) and nitromethane (5.2g, 0.086mol) in 25ml of methanol, add 10% KOH aqueous solution dropwise under stirring at 0-5°C to adjust the pH of the reaction solution to 8-9, continue to react for 2 hours (check the reaction process by TLC), add dropwise 10% KOH aqueous solution to adjust the pH to greater than 10, and react for 30 minutes, quickly pour the reaction solution into ice water with concentrated hydrochloric acid, stir for 30 minutes, add A certain amount of saturated saline, the precip...
Embodiment 2
[0051] Example 2: Take (dl)-trans-4-phenyl-5-o-chlorobenzyl-2-pyrrolidone, grind it through an 80-mesh sieve, add appropriate amount of starch, dextrin, and lactose and mix evenly , granulated, dried, granulated, and divided into capsule shells to obtain capsules. The preparation is orally administered twice a day, and the dose of each dose is 5-100 mg based on the raw material drug.
Embodiment 3
[0052] Example 3: Take 4-phenyl-5-benzyl-2-pyrrolidone, crush it and pass through an 80-mesh sieve, add appropriate amount of starch, dextrin, and lactose, mix evenly by equal multiplication method, granulate, dry, and granulate. The granules are compressed into tablets. The preparation is orally administered twice a day, and the dose of each dose is 5-100 mg based on the raw material drug.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com