Cytotoxicity mediation of cells evidencing surface expression of MCSP
A cytotoxic and cell-based technology, applied in the field of diagnosis and treatment of cancerous diseases, can solve the problems of undisclosed monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
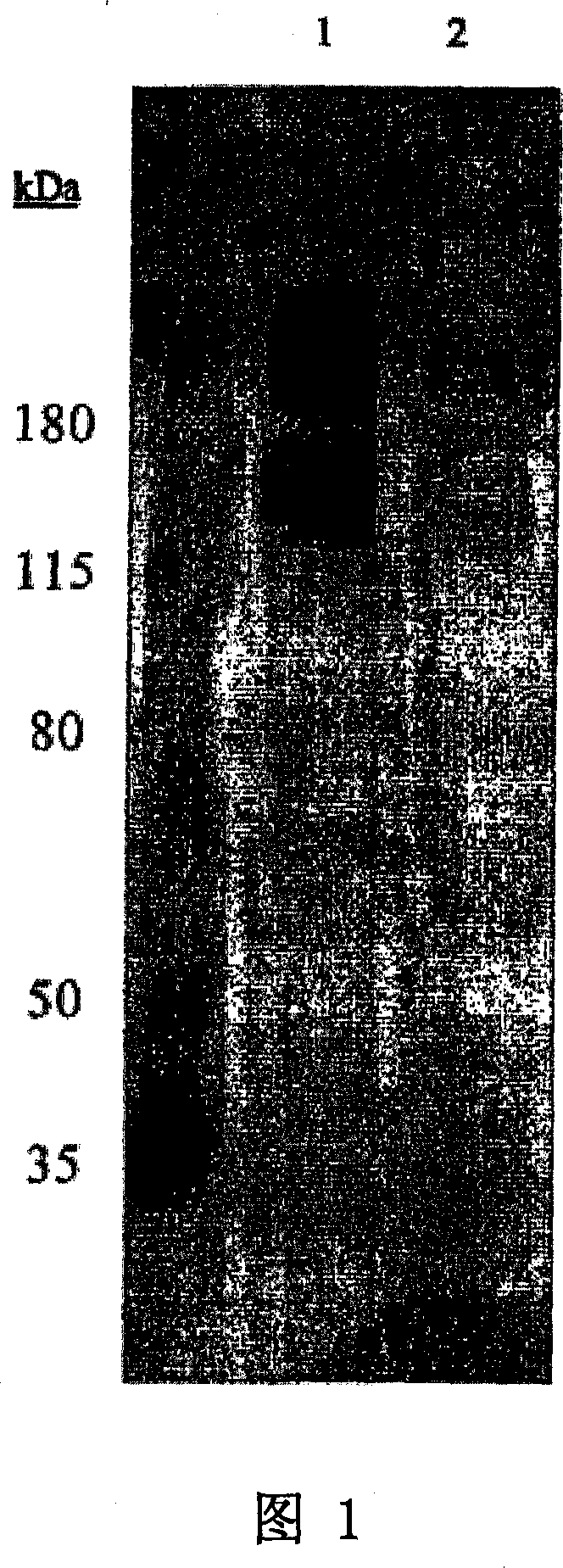
[0075] Identification of binding proteins by Western blotting
[0076] To identify the antigen bound by antibody 11BD-2E11-2, cell membranes expressing the antigen were subjected to gel electrophoresis and transferred to the membrane by Western blotting to determine the proteins detected by the antibody.
[0077] 1: Membrane Preparation
[0078] Previous work has shown that 11BD-2E11-2 binds to the breast cancer cell line MDA-MB-231 (MB-231) by flow cytometry. Previous work also showed the effectiveness of 11BD-2E11-2 against the ovarian cancer cell line OVCAR-3. Therefore, antigen identification was performed using membrane preparations of these two cell lines. Whole-cell membranes were prepared from confluent cultures of MB-231 breast cancer or OVCAR-3 ovarian cells. The medium was removed from the cell stack and the cells were washed with phosphate buffered saline. Cells were dissociated with dissociation buffer (Gibco-BRL, Grand Island, NY) for 20 minutes at 37°C on a ...
Embodiment 2
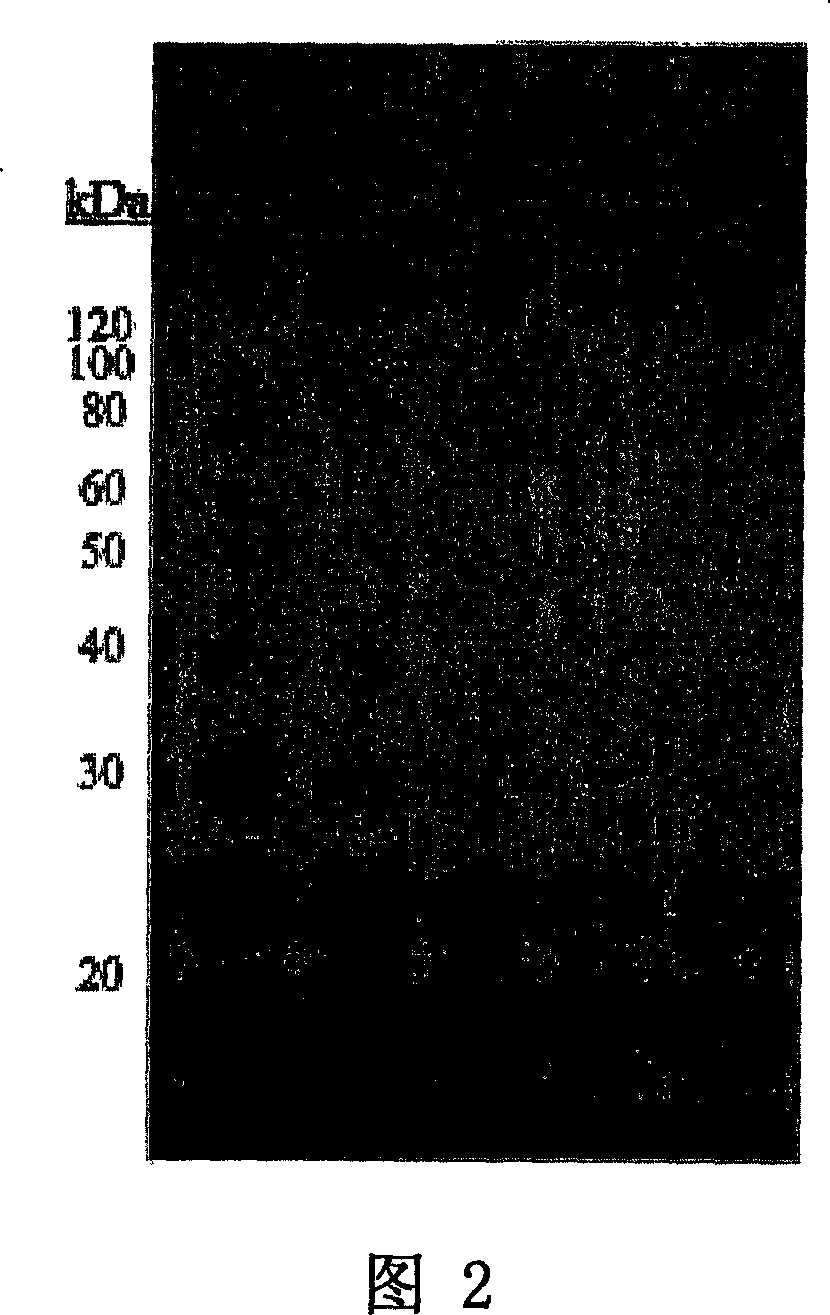
[0082] Determination of glycosylation of antigen bound by 11BD-2E11-2
[0083] To determine whether the antigen recognized by the 11BD-2E11-2 antibody was a glycoprotein, MB-231 membrane protein was treated with different combinations of PNGase F, endo-o-glycosidase, sialidase, galactosidase and glucosamine Glycosidase co-incubation. Membranes were separated by SDS-PAGE and then Western blotted as done with 11BD-2E11-2. Figure 2 shows the results of 11BD-2E11-2 binding to MDA-MB-231 membranes in: a separate deglycosylation buffer (lane 1), with PNGase F, endo- o-glucosidase, sialidase, galactosidase and glucosaminidase (glucosaminidase) in combination (lane 2), and PNG enzyme, endo-o-glucosidase and sialidase in combination (lane 3 ), sialidase alone (lane 4), endo-o-glycosidase alone (lane 5), and PNGase alone (lane 6). Treatment of MB-231 membrane with glycosidase did not eliminate the binding of 11BD-2E11-2, but a shift in protein molecular weight was found in all lanes,...
Embodiment 3
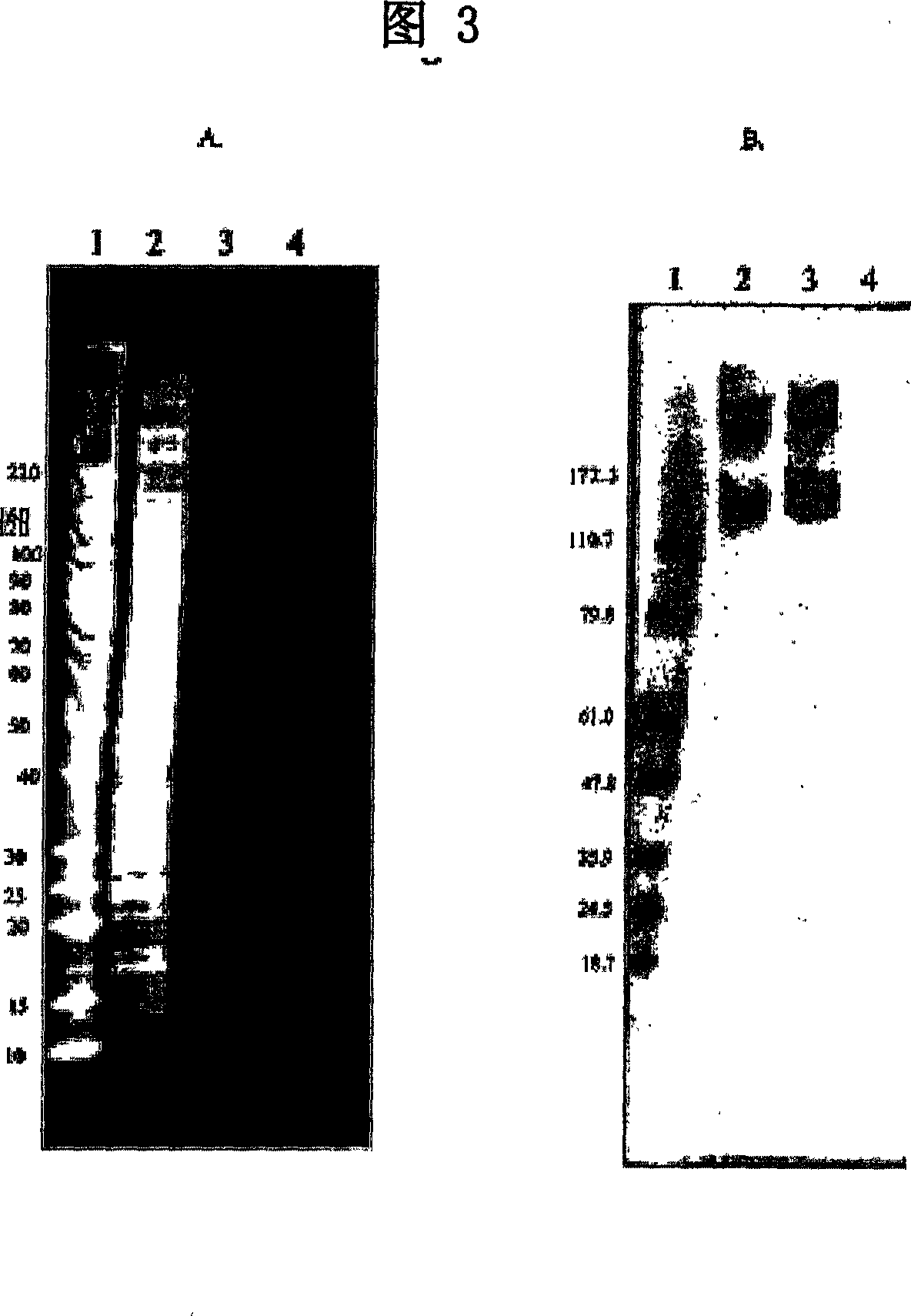
[0085] Identification of 11BD-2E11-2 binding antigen
[0086] 1. Immunoprecipitation
[0087] Cognate ligands were isolated by immunoprecipitation of solubilized membrane glycoproteins with 11BD-2E11-2 antibody to identify the antigen corresponding to 11BD-2E11-2. 100 μL of Protein G Dynabeads (Dynal Biotech, Lake Success NY) were washed 3 times with 1 mL of 0.1 M sodium phosphate buffer, pH 6.0. 100 μg of 11BD-2E11-2 dissolved in pH 6.0 0.1 M sodium phosphate buffer in a total volume of 100 μL was added to the washed beads. The mixture was incubated for 1 hour with rotational mixing. Unbound antibody was removed, and the 11BD-2E11-2-coated antibody was washed three times with 0.5 mL of a pH 7.4 sodium phosphate solution containing 0.1% Tween-20. The 11BD-2E11-2-coated antibody was washed twice with 1 mL of 0.2 M pH 8.2 triethanolamine. 1 mL of 0.02 M dimethylpimelimidate in 0.2 M triethanolamine (pH 8.2) was added and incubated for 30 minutes with swirling mixing to chemi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com